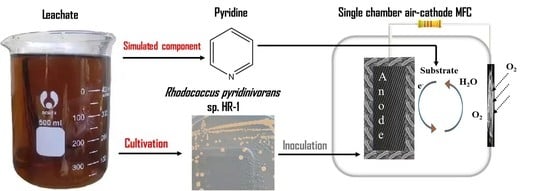
Enhancing Biodegradation of Pyridine with Trehalose Lipid in Rhodococcus pyridinivorans sp. Strain HR-1-Inoculated Microbial Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Separation and Cultivation of Exoelectrogen
2.2. Identification of Strain
2.3. MFC Construction and Operation
2.4. Optimal Operation of Substrate Degradation
2.5. Analytical Methods
3. Results and Discussion
3.1. Isolation, Taxonomy, and Characterization of HR-1 from Leachate-Fed MFC
3.2. Electrical Performance Comparison between Leachate-Fed and HR-1 Inoculated MFC
3.3. Optimization of Substrate Degradation Conditions
3.4. Introduction of Trehalose Lipid to Enhance Pyridine Degradation Rate
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilmasari, D.; Sahabudin, E.; Riyadi, F.A.; Abdullah, N.; Yuzir, A. Future trends and patterns in leachate biological treatment research from a bibliometric perspective. J. Environ. Manag. 2022, 318, 115594. [Google Scholar] [CrossRef] [PubMed]
- Elmaadawy, K.; Liu, B.; Hu, J.; Hou, H.; Yang, J. Performance evaluation of microbial fuel cell for landfill leachate treatment: Research updates and synergistic effects of hybrid systems. J. Environ. Sci. 2020, 96, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Kumar, V.; Malyan, S.K.; Sharma, J.; Mathimani, T.; Maskarenj, M.S.; Ghosh, P.C.; Pugazhendhi, A. Microbial fuel cells (MFCs) for bioelectrochemical treatment of different wastewater streams. Fuel 2019, 254, 115526. [Google Scholar] [CrossRef]
- Mohammadi, M.; Sedighi, M.; Natarajan, R.; Hassan, S.H.A.; Ghasemi, M. Microbial fuel cell for oilfield produced water treatment and reuse: Modelling and process optimization. Korean J. Chem. Eng. 2021, 38, 72–80. [Google Scholar] [CrossRef]
- Kaur, R.; Marwaha, A.; Chhabra, V.A.; Kim, K.-H.; Tripathi, S. Recent developments on functional nanomaterial-based electrodes for microbial fuel cells. Renew. Sust. Energ. Rev. 2020, 119, 109551. [Google Scholar] [CrossRef]
- Ghasemi, M.; Halakoo, E.; Sedighi, M.; Alam, J.; Sadeqzadeh, M. Performance comparison of three common proton exchange membranes for sustainable bioenergy production in microbial fuel cell. Procedia Cirp. 2015, 26, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-H.; Huang, W.-J.; Lin, C.-W.; Zhu, T.-J. Copper removal and microbial community analysis in a single medium sediment microbial fuel cell. J. Water Process Eng. 2021, 44, 102348. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef]
- Ren, S.; Usman, M.; Tsang, D.C.W.; O-Thong, S.; Angelidaki, I.; Zhu, X.; Zhang, S.; Luo, G. Hydrochar-facilitated anaerobic digestion: Evidence for direct interspecies electron transfer mediated through surface oxygen-containing functional groups. Environ. Sci. Technol. 2020, 54, 5755–5766. [Google Scholar] [CrossRef]
- Usman, M.; Shi, Z.; Ren, S.; Ngo, H.H.; Luo, G.; Zhang, S. Hydrochar promoted anaerobic digestion of hydrothermal liquefaction wastewater: Focusing on the organic degradation and microbial community. Chem. Eng. J. 2020, 399, 125766. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Z.; Fu, H.; Zhang, P.; Zheng, Y. Metagenomic analysis of microbial community and gene function of anodic biofilm for nonylphenol removal in microbial fuel cells. J. Clean. Prod. 2022, 374, 133895. [Google Scholar] [CrossRef]
- Usman, M.; Shi, Z.; Ji, M.; Ren, S.; Luo, G.; Zhang, S. Microbial insights towards understanding the role of hydrochar in alleviating ammonia inhibition during anaerobic digestion. Chem. Eng. J. 2021, 419, 129541. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, Y.; Zhang, L.-X.; Zhuang, L.; Zhou, S.-G.; Ni, J.-R. Bioelectricity generation by a Gram-positive Corynebacterium sp. strain MFC03 under alkaline condition in microbial fuel cells. Bioresour. Technol. 2010, 101, 1807–1811. [Google Scholar] [CrossRef]
- Usman, M.; Hao, S.; Chen, H.; Ren, S.; Tsang, D.C.; O-Thong, S.; Luo, G.; Zhang, S. Molecular and microbial insights towards understanding the anaerobic digestion of the wastewater from hydrothermal liquefaction of sewage sludge facilitated by granular activated carbon (GAC). Environ. Int. 2019, 133, 105257. [Google Scholar] [CrossRef]
- Vargas, I.T.; Albert, I.U.; Regan, J.M. Spatial distribution of bacterial communities on volumetric and planar anodes in single-chamber air-cathode microbial fuel cells. Biotechnol. Bioeng. 2013, 110, 3059–3062. [Google Scholar] [CrossRef]
- Lovley, D.R. Extracellular electron transfer: Wires, capacitors, iron lungs, and more. Geobiology 2008, 6, 225–231. [Google Scholar] [CrossRef]
- Lovley, D.R.; Malvankar, N.S. Seeing is believing: Novel imaging techniques help clarify microbial nanowire structure and function. Environ. Microbiol 2015, 17, 2209–2215. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, R.; Chen, J.; Han, W.; Chen, Y.; Wen, Y.; Tang, J. Volatile fatty acids produced by co-fermentation of waste activated sludge and henna plant biomass. Bioresour. Technol. 2016, 211, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Lian, Y.; Yang, Y.; Guo, J.; Wang, Y.; Li, X.; Fang, Y.; Gan, L.; Xu, M. Electron acceptor redox potential globally regulates transcriptomic profiling in Shewanella decolorationis S12. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A. Exoelectrogens: Recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew. Sust. Energ. Rev. 2016, 56, 1322–1336. [Google Scholar] [CrossRef] [Green Version]
- Kokko, M.; Epple, S.; Gescher, J.; Kerzenmacher, S. Effects of wastewater constituents and operational conditions on the composition and dynamics of anodic microbial communities in bioelectrochemical systems. Bioresour. Technol. 2018, 258, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Damiano, L.; Jambeck, J.R.; Ringelberg, D.B. Municipal solid waste landfill leachate treatment and electricity production using microbial fuel cells. Appl. Biochem. Biotechnol. 2014, 173, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jiao, Y.; Lee, D.-J. Transformation of dissolved organic matters in landfill leachate–bioelectrochemical system. Bioresour. Technol. 2015, 191, 350–354. [Google Scholar] [CrossRef]
- Long, J.; Deng, L.; Che, D. Analysis on organic compounds in water leachate from biomass. Renew. Energy 2020, 155, 1070–1078. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Tian, B.-H.; Zhang, X.; Ghulam, A.; Fang, C.-R.; He, R. Investigation on characteristics of leachate and concentrated leachate in three landfill leachate treatment plants. Waste Manag. 2013, 33, 2277–2286. [Google Scholar] [CrossRef]
- Ramírez-Sosa, D.R.; Castillo-Borges, E.R.; Méndez-Novelo, R.I.; Sauri-Riancho, M.R.; Barceló-Quintal, M.; Marrufo-Gómez, J.M. Determination of organic compounds in landfill leachates treated by Fenton–Adsorption. Waste Manag. 2013, 33, 390–395. [Google Scholar] [CrossRef]
- Tripathy, B.K.; Kumar, M. Suitability of microwave and microwave-coupled systems for landfill leachate treatment: An overview. J. Environ. Chem. Eng. 2017, 5, 6165–6178. [Google Scholar] [CrossRef]
- Reis, B.; Silveira, A.; Lebron, Y.; Moreira, V.; Teixeira, L.; Okuma, A.; Amaral, M.; Lange, L. Comprehensive investigation of landfill leachate treatment by integrated Fenton/microfiltration and aerobic membrane bioreactor with nanofiltration. Process Saf. Environ. Prot. 2020, 143, 121–128. [Google Scholar] [CrossRef]
- Li, T.; Song, H.-L.; Xu, H.; Yang, X.-L.; Chen, Q.-L. Biological detoxification and decolorization enhancement of azo dye by introducing natural electron mediators in MFCs. J. Hazard. Mater. 2021, 416, 125864. [Google Scholar] [CrossRef]
- Shi, Z.; Campanaro, S.; Usman, M.; Treu, L.; Basile, A.; Angelidaki, I.; Zhang, S.; Luo, G. Genome-centric metatranscriptomics analysis reveals the role of hydrochar in anaerobic digestion of waste activated sludge. Environ. Sci. Technol. 2021, 55, 8351–8361. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Usman, M.; He, J.; Chen, H.; Zhang, S.; Luo, G. Combined microbial transcript and metabolic analysis reveals the different roles of hydrochar and biochar in promoting anaerobic digestion of waste activated sludge. Water Res. 2021, 205, 117679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; Zhuang, L.; Li, W.; Zhou, S.; Zhang, J. Manganese dioxide as an alternative cathodic catalyst to platinum in microbial fuel cells. Biosens. Bioelectron. 2009, 24, 2825–2829. [Google Scholar] [CrossRef] [PubMed]
- Casella, I.G.; Contursi, M.; Toniolo, R. Anodic electrodeposition of iridium oxide particles on glassy carbon surfaces and their electrochemical/SEM/XPS characterization. J. Electroanal. Chem. 2015, 736, 147–152. [Google Scholar] [CrossRef]
- Ardakani, M.N.; Gholikandi, G.B. Microbial fuel cells (MFCs) in integration with anaerobic treatment processes (AnTPs) and membrane bioreactors (MBRs) for simultaneous efficient wastewater/sludge treatment and energy recovery-A state-of-the-art review. Biomass Bioenergy 2020, 141, 105726. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, Y.; Guo, C.X.; Lim, S.; Song, H.; Li, C.M. Graphene/carbon cloth anode for high-performance mediatorless microbial fuel cells. Bioresour. Technol. 2012, 114, 275–280. [Google Scholar] [CrossRef]
- Okamoto, A.; Hashimoto, K.; Nealson, K.H.; Nakamura, R. Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones. Procl. Natl. Acad. Sci. USA 2013, 110, 7856–7861. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, W.; Li, Y.; Hu, Y.; Zhang, Y. Redox mediator enhanced simultaneous decolorization of azo dye and bioelectricity generation in air-cathode microbial fuel cell. Bioresource Technol. 2013, 142, 407–414. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Kang, S.S.; Cho, Y.G.; Lee, S.T.; Kho, Y.H.; Kim, C.J.; Park, Y.H. Rhodococcus pyridinivorans sp. nov., a pyridine-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2000, 50, 2173–2180. [Google Scholar] [CrossRef]
- Barik, M.; Das, C.P.; Verma, A.K.; Sahoo, S.; Sahoo, N.K. Metabolic profiling of phenol biodegradation by an indigenous Rhodococcus pyridinivorans strain PDB9T N-1 isolated from paper pulp wastewater. Int. Biodeterior. Biodegrad. 2021, 158, 105168. [Google Scholar] [CrossRef]
- Geng, Y.-K.; Yuan, L.; Liu, T.; Li, Z.-H.; Zheng, X.; Sheng, G.-P. In-situ alkaline pretreatment of waste activated sludge in microbial fuel cell enhanced power production. J. Power Sources 2021, 491, 229616. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, J.; Li, J.; Ye, D.; Zhu, X.; Zheng, J.; Zhang, B. Electricity generation and COD removal of microbial fuel cells (MFCs) operated with alkaline substrates. Int. J. Hydrogen Energy 2014, 39, 19349–19354. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, V.; Gude, V.G.; Malyan, S.K.; Pugazhendhi, A. Alkalinity and salinity favor bioelectricity generation potential of Clostridium, Tetrathiobacter and Desulfovibrio consortium in Microbial Fuel Cells (MFC) treating sulfate-laden wastewater. Bioresource Technol. 2020, 306, 123110. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, J.; Zhou, Q.; Gifford, M.; Westerhoff, P. Effects of pH, soluble organic materials, and hydraulic loading rates on orthophosphate recovery from organic wastes using ion exchange. J. Clean. Prod. 2019, 217, 127–133. [Google Scholar] [CrossRef]
- Cheng, P.; Shan, R.; Yuan, H.-R.; Deng, L.-F.; Chen, Y. Enhanced Rhodococcus pyridinivorans HR-1 anode performance by adding trehalose lipid in microbial fuel cell. Bioresource Technol. 2018, 267, 774–777. [Google Scholar] [CrossRef]
- Xiao, N.; Wu, R.; Huang, J.J.; Selvaganapathy, P.R. Anode surface modification regulates biofilm community population and the performance of micro-MFC based biochemical oxygen demand sensor. Chem. Eng. Sci. 2020, 221, 115691. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, P.; Usman, M.; Arslan, M.; Sun, H.; Zhou, L.; Gamal El-Din, M. Enhancing Biodegradation of Pyridine with Trehalose Lipid in Rhodococcus pyridinivorans sp. Strain HR-1-Inoculated Microbial Fuel Cell. Fermentation 2023, 9, 133. https://doi.org/10.3390/fermentation9020133
Cheng P, Usman M, Arslan M, Sun H, Zhou L, Gamal El-Din M. Enhancing Biodegradation of Pyridine with Trehalose Lipid in Rhodococcus pyridinivorans sp. Strain HR-1-Inoculated Microbial Fuel Cell. Fermentation. 2023; 9(2):133. https://doi.org/10.3390/fermentation9020133
Chicago/Turabian StyleCheng, Peng, Muhammad Usman, Muhammad Arslan, Huanqing Sun, Li Zhou, and Mohamed Gamal El-Din. 2023. "Enhancing Biodegradation of Pyridine with Trehalose Lipid in Rhodococcus pyridinivorans sp. Strain HR-1-Inoculated Microbial Fuel Cell" Fermentation 9, no. 2: 133. https://doi.org/10.3390/fermentation9020133
APA StyleCheng, P., Usman, M., Arslan, M., Sun, H., Zhou, L., & Gamal El-Din, M. (2023). Enhancing Biodegradation of Pyridine with Trehalose Lipid in Rhodococcus pyridinivorans sp. Strain HR-1-Inoculated Microbial Fuel Cell. Fermentation, 9(2), 133. https://doi.org/10.3390/fermentation9020133