Comparing VFA Composition, Biomethane Potential, and Methane Production Kinetics of Different Substrates for Anaerobic Fermentation and Digestion
Abstract
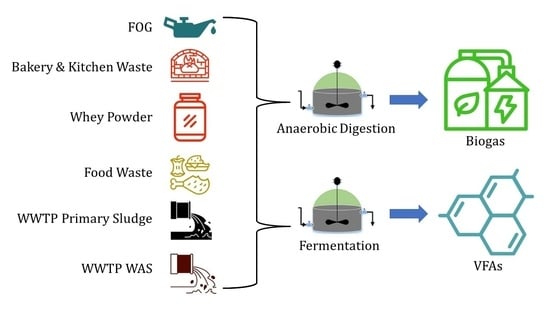
:1. Introduction
2. Methods
2.1. Inoculum
2.2. Substrates
2.3. Substrate Fermentation
2.4. Biochemical Methane Potential
2.5. Biodegradability
2.6. Gompertz Kinetics
2.7. First-Order Kinetics
3. Results
3.1. Fermentation
3.2. Biomethane Production
3.3. Biodegradability
3.4. Kinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Q.; Li, J.; Zeng, X. Minimizing the increasing solid waste through zero waste strategy. J. Clean. Prod. 2015, 104, 199–210. [Google Scholar] [CrossRef]
- Sabour, M.R.; Alam, E.; Hatami, A.M. Global trends and status in landfilling research: A systematic analysis. J. Mater. Cycles Waste Manag. 2020, 22, 711–723. [Google Scholar] [CrossRef]
- Pullen, T. Anaerobic Digestion-Making Biogas-Making Energy; Taylor & Francis Group: Oxford, UK, 2015. [Google Scholar]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Caposciutti, G.; Baccioli, A.; Ferrari, L.; Desideri, U. Biogas from anaerobic digestion: Power generation or biomethane production? Energies 2020, 13, 743. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Demitry, M.E.; Zhong, J.; Hansen, C.L.; McFarland, M.J. Modifying the ADM1 model to predict the operation of an anaerobic digester co-digesting municipal sludge with bakery waste. Environ. Pollut. 2015, 4, 38. [Google Scholar] [CrossRef]
- Chen, J.P.; Yang, L.; Bai, R.; Hung, Y.-T. Bakery waste treatment. In Handbook of Industrial and Hazardous Wastes Treatment; CRC Press: Boca Raton, FL, USA, 2004; pp. 1201–1220. [Google Scholar]
- Salama, E.-S.; Saha, S.; Kurade, M.; Dev, S.; Chang, S.; Jeon, B.-H. Recent trends in anaerobic co-digestion: Fat, oil, and grease (FOG) for enhanced biomethanation. Prog. Energy Combust. Sci. 2019, 70, 22–42. [Google Scholar] [CrossRef]
- Aziz, T.; Holt, L.; Keener, K.; Groninger, J.; Ducoste, J. Performance of grease abatement devices for removal of fat, oil, and grease. J. Environ. Eng. 2011, 137, 84–92. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Cetecioglu, Z. A comprehensive study of volatile fatty acids production from batch reactor to anaerobic sequencing batch reactor by using cheese processing wastewater. Bioresour. Technol. 2020, 311, 123529. [Google Scholar] [CrossRef]
- Lagoa-Costa, B.; Kennes, C.; Veiga, M.C. Cheese whey fermentation into volatile fatty acids in an anaerobic sequencing batch reactor. Bioresour. Technol. 2020, 308, 123226. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile fatty acids production during mixed culture fermentation—The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Zhou, Q.; Song, Z. The effect of pH on anaerobic fermentation of primary sludge at room temperature. J. Hazard. Mater. 2009, 172, 196–201. [Google Scholar] [CrossRef]
- Li, X.; Peng, Y.; Zhao, Y.; Zhang, L.; Han, B. Volatile fatty acid accumulation by alkaline control strategy in anaerobic fermentation of primary sludge. Environ. Eng. Sci. 2017, 34, 703–710. [Google Scholar] [CrossRef]
- Cokgor, E.U.; Oktay, S.; Tas, D.O.; Zengin, G.E.; Orhon, D. Influence of pH and temperature on soluble substrate generation with primary sludge fermentation. Bioresour. Technol. 2009, 100, 380–386. [Google Scholar] [CrossRef]
- Bahreini, G.; Nazari, L.; Ho, D.; Flannery, C.C.; Elbeshbishy, E.; Santoro, D.; Nakhla, G. Enzymatic pre-treatment for enhancement of primary sludge fermentation. Bioresour. Technol. 2020, 305, 123071. [Google Scholar] [CrossRef]
- Huang, X.; Dong, W.; Wang, H.; Sun, F.; Feng, Y. Enhance primary sludge acidogenic fermentation with CaO2 addition: Investigation on soluble substrate generation, sludge dewaterability, and molecular biological characteristics. J. Clean. Prod. 2019, 228, 1526–1536. [Google Scholar] [CrossRef]
- Kurniawan, A.; Kwon, S.; Shin, J.-H.; Hur, J.; Cho, J. Acid fermentation process combined with post denitrification for the treatment of primary sludge and wastewater with high strength nitrate. Water 2016, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- laqa Kakar, F.; Koupaie, E.H.; Razavi, A.S.; Hafez, H.; Elbeshbishy, E. Effect of hydrothermal pretreatment on volatile fatty acids production from thickened waste activated sludge. BioEnergy Res. 2020, 13, 591–604. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Gutiérrez, M.; Garcia-Aguirre, J.; Irizar, I.; Aymerich, E. From sewage sludge and agri-food waste to VFA: Individual acid production potential and up-scaling. Waste Manag. 2018, 77, 203–212. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Shen, D.; Li, N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour. Technol. 2014, 161, 395–401. [Google Scholar] [CrossRef]
- He, X.; Yin, J.; Liu, J.; Chen, T.; Shen, D. Characteristics of acidogenic fermentation for volatile fatty acid production from food waste at high concentrations of NaCl. Bioresour. Technol. 2019, 271, 244–250. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.-C.; Dai, Y.; Tong, Y.W. Acidogenic fermentation of food waste for production of volatile fatty acids: Bacterial community analysis and semi-continuous operation. Waste Manag. 2020, 109, 75–84. [Google Scholar] [CrossRef]
- Ji, Z.; Chen, G.; Chen, Y. Effects of waste activated sludge and surfactant addition on primary sludge hydrolysis and short-chain fatty acids accumulation. Bioresour. Technol. 2010, 101, 3457–3462. [Google Scholar] [CrossRef] [PubMed]
- Ariunbaatar, J.; Panico, A.; Frunzo, L.; Esposito, G.; Lens, P.N.L.; Pirozzi, F. Enhanced anaerobic digestion of food waste by thermal and ozonation pretreatment methods. J. Environ. Manag. 2014, 146, 142–149. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Parthiba Karthikeyan, O.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of food waste for methane and hydrogen recovery: A review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahammad, S.Z.; Gomes, J.; Sreekrishnan, T.R. Wastewater treatment for production of H2S-free biogas. J. Chem. Technol. Biotechnol. 2008, 83, 1163–1169. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Hamza, R.A.; Elbeshbishy, E. Enhancement of denitrification efficiency using municipal and industrial waste fermentation liquids as external carbon sources. Sci. Total Environ. 2022, 816, 151578. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.; Nakhla, G.; El Naggar, M.H.; Elbeshbishy, E.; Baghchehsaraee, B. Effect of organic loading on a novel hydrogen bioreactor. Int. J. Hydrogen Energy 2010, 35, 81–92. [Google Scholar] [CrossRef]
- Bazyar Lakeh, A.A.; Azizi, A.; Hosseini Koupaie, E.; Bekmuradov, V.; Hafez, H.; Elbeshbishy, E. A comprehensive study for characteristics, acidogenic fermentation, and anaerobic digestion of source separated organics. J. Clean. Prod. 2019, 228, 73–85. [Google Scholar] [CrossRef]
- Cioabla, A.E.; Ionel, I.; Dumitrel, G.-A.; Popescu, F. Comparative study on factors affecting anaerobic digestion of agricultural vegetal residues. Biotechnol. Biofuels 2012, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Hafez, H.; Lee, H.-S. Hydrogen production from sugar beet juice using an integrated biohydrogen process of dark fermentation and microbial electrolysis cell. Bioresour. Technol. 2015, 198, 223–230. [Google Scholar] [CrossRef]
- McInerney, M.J. Anaerobic hydrolysis and fermentation of fats and proteins. In Biology of Anaerobic Microorganisms; Zehnder, A.J.B., Ed.; Wiley: New York, NY, USA, 1988; pp. 373–415. [Google Scholar]
- Elefsiniotis, P.; Wareham, D.G.; Smith, M.O. Use of volatile fatty acids from an acid-phase digester for denitrification. J. Biotechnol. 2004, 114, 289–297. [Google Scholar] [CrossRef]
- Yin, J.; Liu, J.; Chen, T.; Long, Y.; Shen, D. Influence of melanoidins on acidogenic fermentation of food waste to produce volatility fatty acids. Bioresour. Technol. 2019, 284, 121–127. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Elefsiniotis, P.; Wareham, D.G. Utilization patterns of volatile fatty acids in the denitrification reaction. Enzym. Microb. Technol. 2007, 41, 92–97. [Google Scholar] [CrossRef]
- Zacharof, M.-P.; Lovitt, R.W. Complex effluent streams as a potential source of volatile fatty acids. Waste Biomass Valorization 2013, 4, 557–581. [Google Scholar] [CrossRef]
- Yin, J.; Yu, X.; Wang, K.; Shen, D. Acidogenic fermentation of the main substrates of food waste to produce volatile fatty acids. Int. J. Hydrogen Energy 2016, 41, 21713–21720. [Google Scholar] [CrossRef]
- Dwidar, M.; Park, J.-Y.; Mitchell, R.J.; Sang, B.-I. The future of butyric acid in industry. Sci. World J. 2012, 2012, 471417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.H.; Aziz, T.N.; de los Reyes, F.L.; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Carbohydrate-enriched cyanobacterial biomass as feedstock for bio-methane production through anaerobic digestion. Fuel 2013, 111, 872–879. [Google Scholar] [CrossRef]
- Okoro-Shekwaga, C.K.; Turnell Suruagy, M.V.; Ross, A.; Camargo-Valero, M.A. Particle size, inoculum-to-substrate ratio and nutrient media effects on biomethane yield from food waste. Renew. Energy 2020, 151, 311–321. [Google Scholar] [CrossRef]
- Cirne, D.G.; Paloumet, X.; Björnsson, L.; Alves, M.M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste—Effects of lipid concentration. Renew. Energy 2007, 32, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Jeganathan, J.; Nakhla, G.; Bassi, A. Long-term performance of high-rate anaerobic reactors for the treatment of oily wastewater. Environ. Sci. Technol. 2006, 40, 6466–6472. [Google Scholar] [CrossRef]
- Palatsi, J.; Laureni, M.; Andrés, M.V.; Flotats, X.; Nielsen, H.B.; Angelidaki, I. Strategies for recovering inhibition caused by long chain fatty acids on anaerobic thermophilic biogas reactors. Bioresour. Technol. 2009, 100, 4588–4596. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Klemeš, J.J.; Ho, C.S.; Ho, W.S. The characterisation and treatment of food waste for improvement of biogas production during anaerobic digestion—A review. J. Clean. Prod. 2018, 172, 1545–1558. [Google Scholar] [CrossRef]







| Substrate | TVFAs | VFA Yield | VFA Fractions (%) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Acetic | Propionic | Butyric | Valeric | Iso-valeric | Other | ||||
| Cheese Whey | 2270 | 55 ** | 43 | 15 | 42 | - | - | - | [12] |
| Cheese Whey | 1140 | 570 * | 22 | 22 | 5 | - | - | 51 | [13] |
| Cheese Whey | 9720 | 2320 | 38 | 20 | 22 | 4 | - | 16 | [14] |
| Cheese Whey | 20,000 | 4000 | 25 | 18 | 20 | 16 | - | 21 | [15] |
| Primary sludge | 2500 | 215 | 44 | 32 | 13 | 4 | 5 | 2 | [16] |
| Primary sludge | 2400 | 206 | 34 | 35 | 14 | 5 | 8 | 4 | [16] |
| Primary sludge | 4000 | 115 | 43 | 26 | 4 | 7 | 6 | 14 | [17] |
| Primary sludge | 3460 | 177 | 70 | 18 | - | - | - | 12 | [18] |
| Primary sludge | 4593 | 201 * | 43 | 33 | 12 | 7 | 2 | 3 | [19] |
| Primary sludge | 800 | 136 | 51 | 19 | 13 | 5 | 7 | 5 | [20] |
| Primary sludge | 1930 | 181 | 30 | 29 | 18 | 10 | 7 | 6 | [21] |
| TWAS | 1800 | - | 22 | 23 | 10 | 17 | 26 | 2 | [22] |
| TWAS | 6000 | 210 * | 28 | 24 | 20 | 11 | 12 | 5 | [23] |
| TWAS | 1150 | 120 | 2 | 53 | - | - | 33 | 12 | [24] |
| Food Waste | 32,000 | 482 | 30 | 15 | 53 | 2 | - | - | [25] |
| Food Waste | 26,610 | 395 * | 65 | 6 | 29 | - | - | - | [26] |
| Food Waste | 2950 | 270 | 37 | - | - | 25 | 9 | 29 | [24] |
| Food Waste | 8682 | 395 * | 43 | 51 | 6 | - | - | - | [27] |
| Substrate | Fermentation | Digestion | ||
|---|---|---|---|---|
| Substrate Volume (mL) | Seed Volume (mL) | Substrate Volume (mL) | Seed Volume (mL) | |
| PS | 572 | 1228 | 64 | 136 |
| TWAS | 514 | 1286 | 57 | 143 |
| FOG | 249 | 1551 | 28 | 172 |
| BP + KW | 245 | 1555 | 27 | 173 |
| FW | 136 | 1664 | 15 | 185 |
| WP | 98 | 1702 | 11 | 189 |
| Substrate | Kinetic Parameters | ||||
|---|---|---|---|---|---|
| P (mL) | Rm (mL/d) | (d) | R2 | k (d−1) | |
| PS | 278 | 49 | 0 | 0.978 | 0.189 |
| TWAS | 238 | 38 | 0 | 0.985 | 0.166 |
| FW | 400 | 67 | 0 | 0.992 | 0.168 |
| BP + KW | 421 | 54 | 0 | 0.989 | 0.117 |
| FOG | 351 | 31 | 0 | 0.98 | 0.263 |
| WP | 433 | 84 | 0 | 0.987 | 0.189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.; Zaghloul, M.S.; Hamza, R.A.; Elbeshbishy, E. Comparing VFA Composition, Biomethane Potential, and Methane Production Kinetics of Different Substrates for Anaerobic Fermentation and Digestion. Fermentation 2023, 9, 138. https://doi.org/10.3390/fermentation9020138
Mahmoud A, Zaghloul MS, Hamza RA, Elbeshbishy E. Comparing VFA Composition, Biomethane Potential, and Methane Production Kinetics of Different Substrates for Anaerobic Fermentation and Digestion. Fermentation. 2023; 9(2):138. https://doi.org/10.3390/fermentation9020138
Chicago/Turabian StyleMahmoud, Ali, Mohamed Sherif Zaghloul, Rania Ahmed Hamza, and Elsayed Elbeshbishy. 2023. "Comparing VFA Composition, Biomethane Potential, and Methane Production Kinetics of Different Substrates for Anaerobic Fermentation and Digestion" Fermentation 9, no. 2: 138. https://doi.org/10.3390/fermentation9020138
APA StyleMahmoud, A., Zaghloul, M. S., Hamza, R. A., & Elbeshbishy, E. (2023). Comparing VFA Composition, Biomethane Potential, and Methane Production Kinetics of Different Substrates for Anaerobic Fermentation and Digestion. Fermentation, 9(2), 138. https://doi.org/10.3390/fermentation9020138