Characterisation of Lacto-Fermented Cricket (Acheta domesticus) Flour and Its Influence on the Quality Parameters and Acrylamide Formation in Wheat Biscuits
Abstract
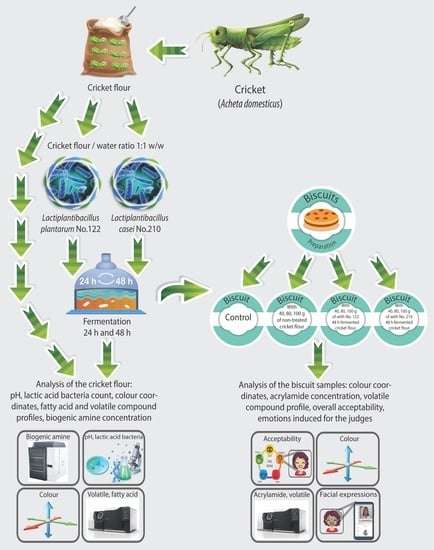
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cricket Flour (Cr) Fermentation
2.3. Analyses of Cricket Flour (Cr)
2.4. Biscuit Formulation and Preparation
2.5. Analysis of Biscuit Samples
2.6. Statistical Analysis
3. Results and Discussion
3.1. pH, Colour Coordinates (a*, b* and L*) and Lactic Acid Bacteria (LAB) Counts in Non-Treated and Fermented Cricket Flour (Cr)
3.2. Biogenic Amine (BA) Concentration in Non-Treated and Fermented Cricket Flour (Cr)
3.3. Fatty Acid (FA) Composition in Cricket Flour (Cr)
3.4. Volatile Compound (VC) Profile in Cricket Flour (Cr)
3.5. Colour Coordinates (a*, b* and L*) and Acrylamide Concentration in Biscuit Samples
3.6. Volatile Compound (VC) Formation in Biscuits and Their Relationship with Acrylamide Content
3.7. Overall Acceptability (OA) and Emotions Induced by Biscuit Samples in the Judge Panel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sorjonen, J. Could Edible Insects Be One Solution for a Circular Economy and Food Production? Designing Feeds for Insects Using Food Industry by-Products; Itä-Suomen yliopisto; University of Eastern Finland: Kuopio, Finland, 2022. [Google Scholar]
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional Value, Protein and Peptide Composition of Edible Cricket Powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-Blind, Randomized Crossover Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lucas, A.J.; de Oliveira, L.M.; Da Rocha, M.; Prentice, C. Edible Insects: An Alternative of Nutritional, Functional and Bioactive Compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Bose, U.; Broadbent, J.A.; Juhász, A.; Karnaneedi, S.; Johnston, E.B.; Stockwell, S.; Byrne, K.; Limviphuvadh, V.; Maurer-Stroh, S.; Lopata, A.L.; et al. Protein Extraction Protocols for Optimal Proteome Measurement and Arginine Kinase Quantitation from Cricket Acheta domesticus for Food Safety Assessment. Food Chem. 2021, 348, 129110. [Google Scholar] [CrossRef] [PubMed]
- Padulo, C.; Carlucci, L.; Balsamo, M.; Fairfield, B. A Dynamic Hop to Cricket Consumption: Factors Influencing Willingness to Try Insect-Based Food. J. Insects Food Feed 2022, 8, 1157–1168. [Google Scholar] [CrossRef]
- Ruszkowska, M.; Tańska, M.; Kowalczewski, P.Ł. Extruded Corn Snacks with Cricket Powder: Impact on Physical Parameters and Consumer Acceptance. Sustainability 2022, 14, 16578. [Google Scholar] [CrossRef]
- Zielińska, E.; Zieliński, D.; Karaś, M.; Jakubczyk, A. Exploration of Consumer Acceptance of Insects as Food in Poland. J. Insects Food Feed 2020, 6, 383–392. [Google Scholar] [CrossRef]
- Kröger, T.; Dupont, J.; Büsing, L.; Fiebelkorn, F. Acceptance of Insect-Based Food Products in Western Societies: A Systematic Review. Front. Nutr. 2022, 8, 759885. [Google Scholar] [CrossRef]
- Florença, S.G.; Guiné, R.P.F.; Gonçalves, F.J.A.; Barroca, M.J.; Ferreira, M.; Costa, C.A.; Correia, P.M.R.; Cardoso, A.P.; Campos, S.; Anjos, O.; et al. The Motivations for Consumption of Edible Insects: A Systematic Review. Foods 2022, 11, 3643. [Google Scholar] [CrossRef]
- Modlinska, K.; Adamczyk, D.; Maison, D.; Goncikowska, K.; Pisula, W. Relationship between Acceptance of Insects as an Alternative to Meat and Willingness to Consume Insect-Based Food—A Study on a Representative Sample of the Polish Population. Foods 2021, 10, 2420. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Kowalczewski, P.Ł.; Drabińska, N.; Różańska, M.B.; Jeleń, H.H. Effect of Cricket Powder Incorporation on the Profile of Volatile Organic Compounds, Free Amino Acids and Sensory Properties of Gluten-Free Bread. Pol. J. Food Nutr. Sci. 2022, 72, 431–442. [Google Scholar] [CrossRef]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten Free Sourdough Bread Enriched with Cricket Flour for Protein Fortification: Antioxidant Improvement and Volatilome Characterization. Food Chem. 2020, 333, 127410. [Google Scholar] [CrossRef] [PubMed]
- Smart Cities Market Report. Size, Share, Growth & Trends (2023–28). Available online: https://www.mordorintelligence.com/industry-reports/smart-cities-market (accessed on 10 January 2023).
- Altemimi, A.B.; Al-Hilphy, A.R.S.; Abedelmaksoud, T.G.; Aboud, S.A.; Badwaik, L.S.; G, L.; Noore, S.; Pratap-Singh, A. Infrared Radiation Favorably Influences the Quality Characteristics of Key Lime Juice. Appl. Sci. 2021, 11, 2842. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Acrylamide. IARC monographs on the evaluation of carcinogenic risk to humans. In Some Industrial Chemicals; WHO Press: Geneva, Switzerland, 1994; Volume 60, Available online: http://monographs.iarc.fr/ENG/Monographs/vol60/volume60.pdf (accessed on 10 January 2023).
- Swedish, N.F.A. Information about Acrylamide in Food. Swed. Natl. Food Adm. 2002, 4, 24. [Google Scholar]
- EU Commission. Commission Regulation 2017/2158 of 20 November 2017 Establishing Mitigation Measures and Benchmark Levels for the Reduction of the Presence of Acrylamide in Food. J. Eur. Union No L 2017, 304, 24. [Google Scholar]
- Schouten, M.A.; Tappi, S.; Glicerina, V.; Rocculi, P.; Angeloni, S.; Cortese, M.; Caprioli, G.; Vittori, S.; Romani, S. Formation of Acrylamide in Biscuits during Baking under Different Heat Transfer Conditions. LWT 2022, 153, 112541. [Google Scholar] [CrossRef]
- Sazesh, B.; Goli, M. Quinoa as a Wheat Substitute to Improve the Textural Properties and Minimize the Carcinogenic Acrylamide Content of the Biscuit. J. Food Process. Preserv. 2020, 44, e14563. [Google Scholar] [CrossRef]
- Žilić, S.; Aktağ, I.G.; Dodig, D.; Filipović, M.; Gökmen, V. Acrylamide Formation in Biscuits Made of Different Wholegrain Flours Depending on Their Free Asparagine Content and Baking Conditions. Food Res. Int. 2020, 132, 109109. [Google Scholar] [CrossRef]
- Schouten, M.A.; Fryganas, C.; Tappi, S.; Romani, S.; Fogliano, V. Influence of Lupin and Chickpea Flours on Acrylamide Formation and Quality Characteristics of Biscuits. Food Chem. 2023, 402, 134221. [Google Scholar] [CrossRef]
- Miśkiewicz, K.; Nebesny, E.; Oracz, J. Formation of Acrylamide during Baking of Shortcrust Cookies Derived from Various Flours. Czech J. Food Sci. 2012, 30, 53–56. [Google Scholar] [CrossRef]
- Miśkiewicz, K.; Rosicka-Kaczmarek, J.; Nebesny, E. Effects of Chickpea Protein on Carbohydrate Reactivity in Acrylamide Formation in Low Humidity Model Systems. Foods 2020, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, P.; Eriksson, S.; Tareke, E.; Karlsson, P.; Ehrenberg, L.; Törnqvist, M. Investigations of Factors That Influence the Acrylamide Content of Heated Foodstuffs. J. Agric. Food Chem. 2003, 51, 7012–7018. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of Acrylamide, a Carcinogen Formed in Heated Foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef] [PubMed]
- Albedwawi, A.S.; Turner, M.S.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Liu, S.-Q.; Shah, N.P.; Ayyash, M.M. An Overview of Microbial Mitigation Strategies for Acrylamide: Lactic Acid Bacteria, Yeast, and Cell-Free Extracts. LWT 2021, 143, 111159. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, C.; Pei, K.; Cai, Y.; Zhang, G.; Hu, C.; Ou, S. Cysteine Alone or in Combination with Glycine Simultaneously Reduced the Contents of Acrylamide and Hydroxymethylfurfural. LWT—Food Sci. Technol. 2015, 63, 275–280. [Google Scholar] [CrossRef]
- Chiş, M.S.; Păucean, A.; Man, S.M.; Vodnar, D.C.; Teleky, B.-E.; Pop, C.R.; Stan, L.; Borsai, O.; Kadar, C.B.; Urcan, A.C.; et al. Quinoa Sourdough Fermented with Lactobacillus Plantarum ATCC 8014 Designed for Gluten-Free Muffins—A Powerful Tool to Enhance Bioactive Compounds. Appl. Sci. 2020, 10, 7140. [Google Scholar] [CrossRef]
- Kewuyemi, Y.O.; Kesa, H.; Chinma, C.E.; Adebo, O.A. Fermented Edible Insects for Promoting Food Security in Africa. Insects 2020, 11, 283. [Google Scholar] [CrossRef]
- Cai, W.; Tang, F.; Zhao, X.; Guo, Z.; Zhang, Z.; Dong, Y.; Shan, C. Different Lactic Acid Bacteria Strains Affecting the Flavor Profile of Fermented Jujube Juice. J. Food Process. Preserv. 2019, 43, e14095. [Google Scholar] [CrossRef]
- Fermented Foods: Past, Present and Future; CRC Press: Boca Raton, FL, USA, 2014; pp. 11–46. ISBN 978-0-429-15716-5.
- Bartkiene, E.; Jomantaite, I.; Mockus, E.; Ruibys, R.; Baltusnikiene, A.; Santini, A.; Zokaityte, E. The Contribution of Extruded and Fermented Wheat Bran to the Quality Parameters of Wheat Bread, Including the Profile of Volatile Compounds and Their Relationship with Emotions Induced for Consumers. Foods 2021, 10, 2501. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Sathyanarayana Rao, T.S.; Yeragani, V.K. Hypertensive Crisis and Cheese. Indian J. Psychiatry 2009, 51, 65–66. [Google Scholar] [CrossRef]
- Santos, M.H.S. Biogenic Amines: Their Importance in Foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Foucher, L.; Barroca, M.J.; Dulyanska, Y.; Correia, P.M.R.; Guiné, R.P.F. Development of Innovative Candied Chestnuts from Three Chestnut Cultivars Grown in Portugal. Foods 2022, 11, 917. [Google Scholar] [CrossRef]
- Bartkiene, E.; Ruzauskas, M.; Lele, V.; Zavistanaviciute, P.; Bernatoniene, J.; Jakstas, V.; Ivanauskas, L.; Zadeike, D.; Klupsaite, D.; Viskelis, P.; et al. Development of Antimicrobial Gummy Candies with Addition of Bovine Colostrum, Essential Oils and Probiotics. Int. J. Food Sci. Technol. 2018, 53, 1227–1235. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Vieites Baptista de Sousa, J.M.; Villa, T.G.; Barros-Velazquez, J. Changes in Biogenic Amines and Microbiological Analysis in Albacore (Thunnus Alalunga) Muscle during Frozen Storage. J. Food Prot. 1998, 61, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Palacios, T.; Ruiz, J.; Ferreira, I.M.P.L.V.O.; Petisca, C.; Antequera, T. Effect of Solvent to Sample Ratio on Total Lipid Extracted and Fatty Acid Composition in Meat Products within Different Fat Content. Meat Sci. 2012, 91, 369–373. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Y.; Ren, Y.; Zhang, Y. Rapid Determination of Acrylamide Contaminant in Conventional Fried Foods by Gas Chromatography with Electron Capture Detector. J. Chromatogr. A 2006, 1116, 209–216. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Duelund, L.; Calleja, G.; Frøst, M.B. Flavour of Fermented Fish, Insect, Game, and Pea Sauces: Garum Revisited. Int. J. Gastron. Food Sci. 2017, 9, 16–28. [Google Scholar] [CrossRef]
- Galli, V.; Venturi, M.; Pini, N.; Granchi, L. Technological Feature Assessment of Lactic Acid Bacteria Isolated from Cricket Powder’s Spontaneous Fermentation as Potential Starters for Cricket-Wheat Bread Production. Foods 2020, 9, 1322. [Google Scholar] [CrossRef] [PubMed]
- American Association of Cereal Chemists; Approved Methods Committee. Approved Methods of the American Association of Cereal Chemists; Amer Assn of Cereal Chemists: Saint Paul, MN, USA, 2000; Volume 1, ISBN 1-891127-12-8. [Google Scholar]
- Romano, A.; Ladero, V.; Alvarez, M.A.; Lucas, P.M. Putrescine Production via the Ornithine Decarboxylation Pathway Improves the Acid Stress Survival of Lactobacillus Brevis and Is Part of a Horizontally Transferred Acid Resistance Locus. Int. J. Food Microbiol. 2014, 175, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.E.; Manca de Nadra, M.C. Biogenic Amine Production by Lactobacillus. J. Appl. Microbiol. 2001, 90, 158–162. [Google Scholar] [CrossRef]
- Bardócz, S. Polyamines in Food and Their Consequences for Food Quality and Human Health. Trends Food Sci. Technol. 1995, 6, 341–346. [Google Scholar] [CrossRef]
- Kalač, P.; Krausová, P. A Review of Dietary Polyamines: Formation, Implications for Growth and Health and Occurrence in Foods. Food Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Marcobal, A.; De Las Rivas, B.; Landete, J.M.; Tabera, L.; Muñoz, R. Tyramine and Phenylethylamine Biosynthesis by Food Bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467. [Google Scholar] [CrossRef]
- La Gioia, F.; Rizzotti, L.; Rossi, F.; Gardini, F.; Tabanelli, G.; Torriani, S. Identification of a Tyrosine Decarboxylase Gene (TdcA) in Streptococcus Thermophilus 1TT45 and Analysis of Its Expression and Tyramine Production in Milk. Appl. Environ. Microbiol. 2011, 77, 1140–1144. [Google Scholar] [CrossRef]
- Wunderlichová, L.; Buňková, L.; Koutný, M.; Jančová, P.; Buňka, F. Formation, Degradation, and Detoxification of Putrescine by Foodborne Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1012–1030. [Google Scholar] [CrossRef]
- Ladero, V.; Sánchez-Llana, E.; Fernández, M.; Alvarez, M.A. Survival of Biogenic Amine-Producing Dairy LAB Strains at Pasteurisation Conditions. Int. J. Food Sci. Technol. 2011, 46, 516–521. [Google Scholar] [CrossRef]
- Vasilica, B.; Tătar, B.; Chiș, M.S.; Alexa, E.; Pop, C.; Păucean, A.; Man, S.; Igual, M.; Haydee, K.M.; Dalma, K.E.; et al. The Impact of Insect Flour on Sourdough Fermentation-Fatty Acids, Amino-Acids, Minerals and Volatile Profile. Insects 2022, 13, 576. [Google Scholar] [CrossRef]
- Williams, J.P.; Williams, J.R.; Kirabo, A.; Chester, D.; Peterson, M. Chapter 3—Nutrient Content and Health Benefits of Insects. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 61–84. ISBN 978-0-12-802856-8. [Google Scholar]
- Castro-López, C.; Santiago-López, L.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Liceaga, A.M.; García, H.S.; Hernández-Mendoza, A. An Insight to Fermented Edible Insects: A Global Perspective and Prospective. Food Res. Int. 2020, 137, 109750. [Google Scholar] [CrossRef]
- Ogawa, J.; Kishino, S.; Ando, A.; Sugimoto, S.; Mihara, K.; Shimizu, S. Production of Conjugated Fatty Acids by Lactic Acid Bacteria. J. Biosci. Bioeng. 2005, 100, 355–364. [Google Scholar] [CrossRef]
- Saeed, A.H.; Salam, A.I. Current Limitations and Challenges with Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2013, 2013, 73–87. [Google Scholar] [CrossRef]
- McFarlane, I.E.; Steeves, E.; Alli, I. Aggregation of Larvae of the House Cricket, Acheta domesticus (L.), by Propionic Acid Present in the Excreta. J. Chem. Ecol. 1983, 9, 1307–1315. [Google Scholar] [CrossRef]
- Xiao, Z.; Xu, P. Acetoin Metabolism in Bacteria. Crit. Rev. Microbiol. 2007, 33, 127–140. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G. Origin and Production of Acetoin during Wine Yeast Fermentation. Appl. Environ. Microbiol. 1996, 62, 309–315. [Google Scholar] [CrossRef]
- Shi, X.; Hao, Z.; Wang, R.; Chen, Z.; Zuo, F.; Wan, Y.; Guo, S. Changes of Hexanal Content in Fermented Soymilk: Induced by Lactic Acid Bacterial Fermentation and Thermal Treatment. J. Food Process. Preserv. 2022, 46, e16555. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, S.K.-C. Selected Odor Compounds in Soymilk As Affected by Chemical Composition and Lipoxygenases in Five Soybean Materials. J. Agric. Food Chem. 2007, 55, 426–431. [Google Scholar] [CrossRef]
- Lorn, D.; Nguyen, T.-K.-C.; Ho, P.-H.; Tan, R.; Licandro, H.; Waché, Y. Screening of Lactic Acid Bacteria for Their Potential Use as Aromatic Starters in Fermented Vegetables. Int. J. Food Microbiol. 2021, 350, 109242. [Google Scholar] [CrossRef]
- Hansen, Å.; Hansen, B. Flavour of Sourdough Wheat Bread Crumb. Z. Lebensm.-Unters. Forsch. 1996, 202, 244–249. [Google Scholar] [CrossRef]
- Liu, X.-S.; Liu, J.-B.; Yang, Z.-M.; Song, H.-L.; Liu, Y.; Zou, T.-T. Aroma-Active Compounds in Jinhua Ham Produced With Different Fermentation Periods. Molecules 2014, 19, 19097–19113. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, L.H. Dried Sausages Fermented with Staphylococcus Xylosus at Different Temperatures and with Different Ingredient Levels—Part I. Chemical and Bacteriological Data. Meat Sci. 1995, 41, 179–191. [Google Scholar] [CrossRef]
- Niu, Y.; Yao, Z.; Xiao, Q.; Xiao, Z.; Ma, N.; Zhu, J. Characterization of the Key Aroma Compounds in Different Light Aroma Type Chinese Liquors by GC-Olfactometry, GC-FPD, Quantitative Measurements, and Aroma Recombination. Food Chem. 2017, 233, 204–215. [Google Scholar] [CrossRef]
- Rasi, S.; Vainio, M.; Blasco, L.; Kahala, M.; Leskinen, H.; Tampio, E. Changes in Volatile Fatty Acid Production and Microbiome during Fermentation of Food Waste from Hospitality Sector. J. Environ. Manag. 2022, 308, 114640. [Google Scholar] [CrossRef]
- Pétel, C.; Onno, B.; Prost, C. Sourdough Volatile Compounds and Their Contribution to Bread: A Review. Trends Food Sci. Technol. 2017, 59, 105–123. [Google Scholar] [CrossRef]
- Pop, A.; Păucean, A.; Socaci, S.A.; Alexa, E.; Man, S.M.; Mureșan, V.; Chiş, M.S.; Salanță, L.; Popescu, I.; Berbecea, A.; et al. Quality Characteristics and Volatile Profile of Macarons Modified with Walnut Oilcake By-Product. Molecules 2020, 25, 2214. [Google Scholar] [CrossRef]
- Suman, M.; Generotti, S.; Cirlini, M.; Dall’Asta, C. Acrylamide Reduction Strategy in Combination with Deoxynivalenol Mitigation in Industrial Biscuits Production. Toxins 2019, 11, 499. [Google Scholar] [CrossRef]
- González-Gómez, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Simultaneous Determination of Furanic Compounds and Acrylamide in Insect-Based Foods by HPLC-QqQ-MS/MS Employing a Functionalized Mesostructured Silica as Sorbent in Solid-Phase Extraction. Foods 2021, 10, 1557. [Google Scholar] [CrossRef]
- Chu, F.L.; Yaylayan, V.A. Model Studies on the Oxygen-Induced Formation of Benzaldehyde from Phenylacetaldehyde Using Pyrolysis GC-MS and FTIR. J. Agric. Food Chem. 2008, 56, 10697–10704. [Google Scholar] [CrossRef]
- Elmore, J.S.; Dodson, A.T.; Muttucumaru, N.; Halford, N.G.; Parry, M.A.J.; Mottram, D.S. Effects of Sulphur Nutrition during Potato Cultivation on the Formation of Acrylamide and Aroma Compounds during Cooking. Food Chem. 2010, 122, 753–760. [Google Scholar] [CrossRef]
- Holdeman, L.V.; Moore, W.E.C.; Cato, E.P. Anaerobe Laboratory Manual; Anaerobe Laboratory, Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 1977. [Google Scholar]
- Guarrasi, V.; Sannino, C.; Moschetti, M.; Bonanno, A.; Di Grigoli, A.; Settanni, L. The Individual Contribution of Starter and Non-Starter Lactic Acid Bacteria to the Volatile Organic Compound Composition of Caciocavallo Palermitano Cheese. Int. J. Food Microbiol. 2017, 259, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Shaker, E.S.; Ghazy, M.A.; Shibamoto, T. Antioxidative Activity of Volatile Browning Reaction Products and Related Compounds in a Hexanal/Hexanoic Acid System. J. Agric. Food Chem. 1995, 43, 1017–1022. [Google Scholar] [CrossRef]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the Apple Cultivar on Cloudy Apple Juice Fermented by a Mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 2021, 340, 127922. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Peris, J.E.; Redondo, A.; Shimada, T.; Costell, E.; Carbonell, I.; Rojas, C.; Peña, L. Impact of D-Limonene Synthase up- or down-Regulation on Sweet Orange Fruit and Juice Odor Perception. Food Chem. 2017, 217, 139–150. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Substances Added to Food. Retrieved March 2018, 6, 2020. [Google Scholar]
- Li, Q.; Zhu, X.; Xie, Y.; Liang, J. Antifungal Properties and Mechanisms of Three Volatile Aldehydes (Octanal, Nonanal and Decanal) on Aspergillus flavus. Grain Oil Sci. Technol. 2021, 4, 131–140. [Google Scholar] [CrossRef]
- Vitaglione, P.; Troise, A.D.; De Prisco, A.C.; Mauriello, G.L.; Gokmen, V.; Fogliano, V. Chapter 15—Use of Microencapsulated Ingredients in Bakery Products: Technological and Nutritional Aspects. In Microencapsulation and Microspheres for Food Applications; Sagis, L.M.C., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 301–311. ISBN 978-0-12-800350-3. [Google Scholar]
- Tomasino, E.; Harrison, R.; Breitmeyer, J.; Sedcole, R.; Sherlock, R.; Frost, A. Aroma Composition of 2-Year-Old New Zealand Pinot Noir Wine and Its Relationship to Sensory Characteristics Using Canonical Correlation Analysis and Addition/Omission Tests. Aust. J. Grape Wine Res. 2015, 21, 376–388. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V. What Governs Selection and Acceptance of Edible Insect Species? In Edible Insects in Sustainable Food Systems; Springer: Berlin/Heidelberg, Germany, 2018; pp. 331–351. ISBN 978-3-319-74010-2. [Google Scholar]
- Yazici, G.N.; Ozer, M.S. Using Edible Insects in the Production of Cookies, Biscuits, and Crackers: A Review. Biol. Life Sci. Forum 2021, 6, 80. [Google Scholar]
- Bas, A.; El, S.N. Nutritional Evaluation of Biscuits Enriched with Cricket Flour (Acheta Domesticus). Int. J. Gastron. Food Sci. 2022, 29, 100583. [Google Scholar] [CrossRef]
- Biró, B.; Sipos, M.A.; Kovács, A.; Badak-Kerti, K.; Pásztor-Huszár, K.; Gere, A. Cricket-Enriched Oat Biscuit: Technological Analysis and Sensory Evaluation. Foods 2020, 9, 1561. [Google Scholar] [CrossRef]
- Tedjakusuma, F.; Linggadiputra, J.; Cahya, A.D.; Surya, R. Development of Cricket Flour-Enriched Cookies. IOP Conf. Ser. Earth Environ. Sci. 2022, 1115, 012092. [Google Scholar] [CrossRef]
- Castro Delgado, M.; Chambers, E., IV; Carbonell-Barrachina, A.; Noguera Artiaga, L.; Vidal Quintanar, R.; Burgos Hernandez, A. Consumer Acceptability in the USA, Mexico, and Spain of Chocolate Chip Cookies Made with Partial Insect Powder Replacement. J. Food Sci. 2020, 85, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Gurdian, C.E.; Torrico, D.D.; Li, B.; Tuuri, G.; Prinyawiwatkul, W. Effect of Disclosed Information on Product Liking, Emotional Profile, and Purchase Intent: A Case of Chocolate Brownies Containing Edible-Cricket Protein. Foods 2021, 10, 1769. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Marcia, J.; Pournaki, S.K.; Borrás-Linares, I.; Lozano-Sanchez, J.; Fernandez, I.M. Formulation of Protein-Rich Chocolate Chip Cookies Using Cricket (Acheta Domesticus) Powder. Foods 2022, 11, 3275. [Google Scholar] [CrossRef]
- Pambo, K.O.; Okello, J.J.; Mbeche, R.M.; Kinyuru, J.N.; Alemu, M.H. The Role of Product Information on Consumer Sensory Evaluation, Expectations, Experiences and Emotions of Cricket-Flour-Containing Buns. Food Res. Int. Ott. Ont 2018, 106, 532–541. [Google Scholar] [CrossRef]

| Samples | pH | LAB Counts, log10 CFU/g | Colour Coordinates | ΔE | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| CoCr | 5.94 ± 0.43 c | na | 53.4 ± 1.9 b | 2.25 ± 0.13 a | 15.7 ± 1.2 b | --- |
| 24 h Cr122 | 5.15 ± 0.03 b | 11.6 ± 0.90 b | 35.1 ± 1.28 a | 2.20 ± 0.12 a | 7.13 ± 0.32 a | 18.75 ± 0.24 a |
| 48 h Cr122 | 4.26 ± 0.04 a | 11.8 ± 1.0 b | 36.3 ± 1.12 a | 2.12 ± 0.17 a | 8.24 ± 0.41 a | 17.54 ± 0.37 a |
| 24 h Cr210 | 5.02 ± 0.04 b | 8.29 ± 0.63 a | 32.6 ± 1.93 a | 1.95 ± 0.16 a | 6.99 ± 0.41 a | 22.08 ± 0.79 b |
| 48 h Cr210 | 4.42 ± 0.62 a | 8.44 ± 0.71 a | 32.7 ± 2.13 a | 1.97 ± 0.13 a | 8.11 ± 0.39 a | 21.94 ± 0.89 b |
| Biogenic Amine Concentration, mg/kg | Samples | ||||
|---|---|---|---|---|---|
| CoCr | Cr122 | Cr210 | |||
| Duration of Fermentation, h | |||||
| 0 | 24 | 48 | 24 | 48 | |
| Tryptamine (TRP) | 45.87 ± 4.75 c | 27.31 ± 3.55 b | 20.16 ± 1.99 a | 29.11 ± 5.51 b | 40.83 ± 5.56 c |
| Phenylethylamine (PHE) | 30.16 ± 2.25 b | 20.67 ± 2.25 a | 40.76 ± 2.85 c | 21.03 ± 2.58 a | 50.17 ± 6.09 d |
| Putrescine (PUT) | 40.77 ± 7.08 c | 46.22 ± 7.66 c | 15.15 ± 2.38 a | 56.98 ± 6.13 c | 23.44 ± 2.98 b |
| Cadaverine (CAV) | 69.34 ± 5.76 c | 35.83 ± 3.91 b | nd | 21.89 ± 1.37 a | nd |
| Histamine (HIS) | nd | nd | nd | nd | nd |
| Tyramine (TYR) | 15.4 ± 1.70 a | 199.56 ± 23.2 b | 291.03 ± 42.5 c | 455.01 ± 35.4 d | 443.87 ± 23.0 d |
| Spermidine (SPRMD) | 284.72 ± 37.6 b | 138.32 ± 11.5 a | 150.31 ± 18.7 a | 116.43 ± 11.7 a | 111.02 ± 19.6 a |
| Spermine (SPRM) | 307.19 ± 27.8 b | 161.68 ± 15.6 a | 172.21 ± 24.4 a | 146.02 ± 17.4 a | 147.91 ± 19.6 a |
| Fatty Acid Composition, % | Samples | ||||
|---|---|---|---|---|---|
| CoCr | Cr122 | Cr210 | |||
| Duration of Fermentation, h | |||||
| 0 | 24 | 48 | 24 | 48 | |
| C16:0 | 26.5 ± 0.21 b | 26.8 ± 0.29 b | 24.6 ± 0.42 a | 24.3 ± 0.12 a | 24.7 ± 0.78 a |
| C16:1 | 0.138 ± 0.010 | nd | nd | nd | nd |
| C18:0 | 8.72 ± 0.20 b | 8.40 ± 0.29 b | 7.81 ± 0.10 a | 7.31 ± 0.32 a | 7.65 ± 0.72 a,b |
| C18:1 cis, trans | 27.4 ± 0.11 a | 28.9 ± 0.17 b | 30.5 ± 0.28 c | 31.7 ± 0.53 d | 30.0 ± 0.27 c |
| C18:2 | 35.7 ± 0.31 c | 33.8 ± 0.94 a | 35.2 ± 0.30 a | 33.9 ± 0.18 a | 34.7 ± 0.33 b |
| C18:3 α | 1.52 ± 0.02 a | 1.99 ± 0.02 b | 1.92 ± 0.05 b | 2.71 ± 0.19 c | 2.98 ± 0.16 c |
| Retention Time (RT), min | Volatile Compounds | Samples | ||||
|---|---|---|---|---|---|---|
| CoCr | Cr122 | Cr210 | ||||
| Duration of Fermentation, h | ||||||
| 0 | 24 | 48 | 24 | 48 | ||
| 2.315 | Acetic acid | 17.7 ± 2.39 b | 6.82 ± 1.39 a | 7.76 ± 1.19 a | 19.6 ± 2.01 b | 8.48 ± 1.26 a |
| 3.754 | Acetoin | nd | 28.3 ± 5.44 b | nd | 7.90 ± 0.71 a | nd |
| 5.926 | Hexanal | 15.7 ± 2.33 c | 3.46 ± 0.60 a | nd | 5.98 ± 0.92 b | nd |
| 7.861 | 3-Methylbutanoic acid | nd | 23.6 ± 4.53 d | 7.52 ± 0.96 b | 14.2 ± 2.95 c | 3.99 ± 0.62 a |
| 8.089 | 1-Hexanol | nd | 4.09 ± 0.91 a | nd | 9.35 ± 1.01 b | nd |
| 8.765 | 2-Heptanone | 6.18 ± 1.06 b | 2.45 ± 0.49 a | 35.4 ± 4.82 d | 2.10 ± 0.23 a | 17.5 ± 2.51 c |
| 12.493 | Decane | 28.1 ± 5.65 d | 7.34 ± 0.78 a | 13.3 ± 1.28 b | 10.4 ± 1.20 b | 16.3 ± 1.50 c |
| 18.967 | Dodecane | 9.75 ± 1.88 b | 2.87 ± 0.52 a | 4.10 ± 0.75 a | 4.32 ± 0.82 a | 5.05 ± 0.97 a |
| Biscuit Samples | Colour Coordinates, NBS | Acrylamide Content, µg/kg | |||
|---|---|---|---|---|---|
| L* | a* | b* | ΔE | ||
| C | 67.8 ± 2.22 f | 9.06 ± 1.31 d | 28.4 ± 0.31 e | 302.4 ± 24.3 e | |
| C-CF40 | 58.3 ± 0.52 c | 4.57 ± 0.11 a | 19.8 ± 0.37 a | 13.58 ± 0.27 b,c | 148.2 ± 11.9 b |
| C-CF40-122 | 60.2 ± 0.40 d | 7.47 ± 0.53 c | 22.7 ± 0.19 c,d | 9.63 ± 0.38 a | 192.0 ± 13.5 d |
| C-CF40-210 | 67.0 ± 0.63 f | 4.82 ± 0.27 a | 22.4 ± 0.22 c | 7.39 ± 0.43 a | 84.1 ± 7.20 a |
| C-CF80 | 46.1 ± 0.39 a | 7.23 ± 0.38 c | 23.0 ± 0.24 d | 22.44 ± 0.61 d | 165.4 ± 12.3 c |
| C-CF80-122 | 59.9 ± 0.88 d | 6.38 ± 0.46 b | 21.0 ± 0.37 b | 11.15 ± 0.29 b | 140.3 ± 11.0 b |
| C-CF80-210 | 61.3 ± 0.36 e | 4.60 ± 0.24 a | 19.9 ± 0.37 a | 11.59 ± 0.57 b | 142.1 ± 10.9 b |
| C-CF100 | 47.6 ± 1.13 a | 6.05 ± 0.43 b | 22.3 ± 0.19 c | 21.31 ± 0.47 d | 172.0 ± 14.6 c |
| C-CF100-122 | 58.9 ± 0.28 c | 5.84 ± 0.39 b | 20.1 ± 0.25 a | 12.59 ± 0.14 b | 145.6 ± 13.8 b |
| C-CF100-210 | 55.9 ± 1.13 b | 6.44 ± 0.42 b | 19.8 ± 0.14 a | 14.91 ± 0.21 c | 156.5 ± 14.6 b,c |
 | |||||
 | |||||
| Volatile Compound | RT (min) | C | C-CF40 | C-CF40-122 | C-CF40-210 | C-CF80 | C-CF80-122 | C-CF80-210 | C-CF100 | C-CF100-122 | C-CF100-210 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzaldehyde | 11.8 | 0.98 ± 0.025 a | 1.65 ± 0.19 b | 1.84 ± 0.17 c | 1.67 ± 0.17 b | 1.51 ± 0.14 b | 1.93 ± 0.18 c | 1.76 ± 0.16 b | 1.77 ± 0.18 b | 1.66 ± 0.15 b | 2.01 ± 0.19 c |
| Hexanoic acid | 12.51 | 1.1 ± 0.12 a | 2.71 ± 0.24 b | 2.46 ± 0.23 b | 2.81 ± 0.28 b | 2.87 ± 0.27 b | 3.21 ± 0.29 c | 3.46 ± 0.32 c | 3.41 ± 0.32 c | 3.74 ± 0.29 d | 3.21 ± 0.31 c |
| D-Limonene | 14.09 | 0.099 ± 0.011 a | nd | 1.52 ± 0.14 d | 0.219 ± 0.019 b | 0.235 ± 0.022 b | 0.196 ± 0.18 a | 0.578 ± 0.042 c | 2.23 ± 0.21 e | 2.36 ± 0.19 e | 0.304 ± 0.28 a |
| Phenylacetaldehyde | 14.53 | 1.76 ± 0.016 a | 2.62 ± 0.25 b | 2.01 ± 0.18 a | 2.43 ± 0.23 b | 4.11 ± 0.39 c | 2.61 ± 0.25 b | 2.78 ± 0.26 b | 1.71 ± 0.16 a | 1.9 ± 0.17 a | 2.39 ± 0.22 b |
| (E)-2-Octen-1-al | 15 | 0.283 ± 0.03 d | 0.225 ± 0.021 c | 0.183 ± 0.014 b | 0.189 ± 0.017 b | 0.155 ± 0.014 a | 0.219 ± 0.02 c | 0.275 ± 0.024 d | 0.3 ± 0.022 d | 0.3 ± 0.026 d | 0.223 ± 0.21 c |
| Sorbic acid | 16.36 | 86.3 ± 9.32 a | 81.1 ± 7.85 a | 81.3 ± 6.98 a | 81.7 ± 7.82 a | 81.3 ± 7.52 a | 81 ± 6.89 a | 79.9 ± 7.81 a | 79.6 ± 7.67 a | 78.3 ± 7.58 a | 78.6 ± 7.45 a |
| Nonanal | 16.44 | 0.193 ± 0.023 a | 0.921 ± 0.052 d | 0.76 ± 0.32 c | 0.748 ± 0.041 c | 0.771 ± 0.038 c | 0.806 ± 0.051 c | 0.832 ± 0.059 d | 0.651 ± 0.041 b | 0.733 ± 0.028 c | 0.73 ± 0.38 c |
| Non-(2E)-enal | 18.16 | 0.351 ± 0.04 b | 0.321 ± 0.029 a | 0.375 ± 0.18 c | 0.423 ± 0.036 c | 0.4 ± 0.027 c | 0.476 ± 0.036 d | 0.483 ± 0.03 d | 0.396 ± 0.021 c | 0.439 ± 0.028 c | 0.473 ± 0.031 d |
| Octanoic acid | 18.68 | 4.27 ± 0.32 a | 4.89 ± 0.41 a | 4.7 ± 0.43 a | 4.85 ± 0.35 a | 4.52 ± 0.41 a | 4.56 ± 0.36 a | 4.6 ± 0.28 a | 4.52 ± 0.42 a | 4.71 ± 0.31 a | 4.82 ± 0.027 a |
| Dodecane | 19.36 | 1.12 ± 0.25 b | 0.942 ± 0.036 b | 1.04 ± 0.09 b | 1.22 ± 0.12 c | 1.2 ± 0.11 c | 1.08 ± 0.09 c | 0.854 ± 0.041 a | 1.01 ± 0.1 b | 1.46 ± 0.15 d | 1.22 ± 0.11 c |
| Decanal | 19.52 | 0.226 ± 0.018 b | 0.238 ± 0.021 b | 0.266 ± 0.022 b | 0.204 ± 0.018 a | 0.19 ± 0.018 a | 0.239 ± 0.018 b | 0.243 ± 0.022 b | 0.221 ± 0.019 a | 0.251 ± 0.022 b | 0.344 ± 0.018 c |
| 4,6-dimethyldodecane | 21.68 | 0.34 ± 0.026 f | 0.347 ± 0.031 f | 0.302 ± 0.027 f | 0.082 ± 0.008 b | 0.03 ± 0.004 a | 0.184 ± 0.017 d | 0.299 ± 0.019 f | 0.253 ± 0.023 e | 0.219 ± 0.019 e | 0.152 ± 0.012 c |
| Undecan-2-one | 22.04 | 0.688 ± 0.031 b | 0.994 ± 0.043 e | 0.825 ± 0.035 c | 0.705 ± 0.025 b | 0.45 ± 0.032 a | 0.786 ± 0.036 c | 0.8 ± 0.037 c | 0.8 ± 0.041 c | 0.8 ± 0.031 c | 0.876 ± 0.036 d |
| 5-hexyldihydro-2(3H)-Furanone | 23.97 | 0.398 ± 0.022 a | 0.596 ± 0.039 c | 0.457 ± 0.028 b | 0.45 ± 0.014 b | 0.41 ± 0.028 a | 0.413 ± 0.039 a | 0.514 ± 0.028 b | 0.499 ± 0.031 b | 0.501 ± 0.045 b | 0.462 ± 0.033 b |
| 1-Tetradecene | 24.69 | 0.267 ± 0.023 b | 0.501 ± 0.034 e | 0.284 ± 0.019 b | 0.487 ± 0.027 e | 0.52 ± 0.035 e | 0.37 ± 0.022 d | 0.265 ± 0.024 a | 0.41 ± 0.02 d | 0.4 ± 0.029 d | 0.324 ± 0.027 c |
| Vanillin | 24.96 | 0.746 ± 0.041 c | 0.824 ± 0.027 d | 0.562 ± 0.023 a | 0.66 ± 0.024 b | 0.6 ± 0.041 a | 0.816 ± 0.032 c | 1.06 ± 0.11 e | 0.853 ± 0.034 d | 0.829 ± 0.043 c | 2.66 ± 0.025 f |
| Ethyl dodecanoate | 29.69 | 0.848 ± 0.039 a | 1.15 ± 0.12 b | 1.15 ± 0.12 b | 1.17 ± 0.11 b | 1.18 ± 0.1 b | 1.1 ± 0.11 b | 1.22 ± 0.12 b | 1.37 ± 0.14 b | 1.39 ± 0.12 b | 1.18 ± 0.12 b |
| Biscuit Samples | OA | Emotions Induced by Biscuit Samples in the Judge Panel (from 0 to 1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Neutral | Happy | Sad | Angry | Surprised | Scared | Disgusted | Contempt | ||
| C | 7.5 ± 2.3 a | 0.811 ± 0.079 a | 0.036 ± 0.004 c | 0.020 ± 0.002 c | 0.019 ± 0.002 b,c | 0.038 ± 0.004 e | 0.0008 ± 0.0002 c,d | 0.006 ± 0.001 b | 0.0010 ± 0.0003 a |
| C-CF40 | 6.3 ± 3.1 a | 0.731 ± 0.061 a | 0.059 ± 0.005 d | 0.064 ± 0.006 e | 0.052 ± 0.005 d | 0.033 ± 0.003 d,e | 0.0006 ± 0.0001 b,c | 0.019 ± 0.003 d | 0.0010 ± 0.0002 a |
| C-CF40-122 | 9.1 ± 0.9 a | 0.845 ± 0.078 a | 0.041 ± 0.004 c | 0.014 ± 0.002 b | 0.061 ± 0.006 d | 0.052 ± 0.005 f | 0.0006 ± 0.0002 b,c | 0.005 ± 0.001 b | 0.0010 ± 0.0003 a |
| C-CF40-210 | 7.9 ± 2.5 a | 0.799 ± 0.075 a | 0.036 ± 0.004 c | 0.008 ± 0.001 a | 0.024 ± 0.002 c | 0.054 ± 0.006 f | 0.0006 ± 0.0001 b,c | 0.007 ± 0.002 b,c | 0.0020 ± 0.0004 b |
| C-CF80 | 6.5 ± 1.7 a | 0.706 ± 0.069 a | 0.043 ± 0.004 c | 0.028 ± 0.003 d | 0.015 ± 0.001 b | 0.023 ± 0.002 c | 0.0011 ± 0.0003 d | 0.029 ± 0.003 e | 0.0020 ± 0.0004 b |
| C-CF80-122 | 7.0 ± 1.9 a | 0.807 ± 0.075 a | 0.084 ± 0.007 e | 0.032 ± 0.003 d | 0.017 ± 0.002 b,c | 0.028 ± 0.003 c,d | 0.0017 ± 0.0002 e | 0.017 ± 0.004 d | 0.0020 ± 0.0005 b |
| C-CF80-210 | 6.4 ± 2.3 a | 0.755 ± 0.074 a | 0.020 ± 0.002 a | 0.013 ± 0.001 b | 0.022 ± 0.003 c | 0.051 ± 0.005 f | 0.0002 ± 0.0001 a | 0.009 ± 0.002 b,c | 0.0030 ± 0.0002 c |
| C-CF100 | 5.3 ± 2.5 a | 0.825 ± 0.079 a | 0.023 ± 0.003 a | 0.014 ± 0.002 b | 0.010 ± 0.001 a | 0.012 ± 0.001 a | 0.0004 ± 0.0001 a,b | 0.008 ± 0.001 b,c | 0.0010 ± 0.0002 a |
| C-CF100-122 | 6.3 ± 2.5 a | 0.885 ± 0.081 a | 0.043 ± 0.004 c | 0.017 ± 0.001 b,c | 0.009 ± 0.001 a | 0.026 ± 0.003 c | 0.0006 ± 0.0002 b | 0.010 ± 0.002 c | 0.0010 ± 0.0001 a |
| C-CF100-210 | 7.1 ± 2.6 a | 0.863 ± 0.084 a | 0.028 ± 0.003 a,b | 0.009 ± 0.001 a | 0.024 ± 0.002 c | 0.018 ± 0.002 b | 0.0015 ± 0.0003 d,e | 0.002 ± 0.001 a | 0.0020 ± 0.0005 b |
| Parameters | Factors and Their Interaction | ||
|---|---|---|---|
| Quantity of the Cricket Flour | LAB Strain Used for Cricket Flour Fermentation | Amount of the Cricket Flour Interaction with LAB Strain Used for Cricket Flour Fermentation | |
| Overall acceptability (OA) | 0.814 | 0.583 | 0.947 |
| Neutral | 0.331 | 0.213 | 0.742 |
| Happy | 0.003 | 0.0001 | 0.0001 |
| Sad | 0.036 | 0.004 | 0.068 |
| Angry | 0.0001 | 0.038 | 0.0001 |
| Surprised | 0.001 | 0.013 | 0.027 |
| Scared | 0.005 | 0.043 | 0.0001 |
| Disgusted | 0.0001 | 0.0001 | 0.0001 |
| Contempt | 0.370 | 0.228 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkiene, E.; Zokaityte, E.; Kentra, E.; Starkute, V.; Klupsaite, D.; Mockus, E.; Zokaityte, G.; Cernauskas, D.; Rocha, J.M.; Guiné, R.P.F. Characterisation of Lacto-Fermented Cricket (Acheta domesticus) Flour and Its Influence on the Quality Parameters and Acrylamide Formation in Wheat Biscuits. Fermentation 2023, 9, 153. https://doi.org/10.3390/fermentation9020153
Bartkiene E, Zokaityte E, Kentra E, Starkute V, Klupsaite D, Mockus E, Zokaityte G, Cernauskas D, Rocha JM, Guiné RPF. Characterisation of Lacto-Fermented Cricket (Acheta domesticus) Flour and Its Influence on the Quality Parameters and Acrylamide Formation in Wheat Biscuits. Fermentation. 2023; 9(2):153. https://doi.org/10.3390/fermentation9020153
Chicago/Turabian StyleBartkiene, Elena, Egle Zokaityte, Evaldas Kentra, Vytaute Starkute, Dovile Klupsaite, Ernestas Mockus, Gintare Zokaityte, Darius Cernauskas, João Miguel Rocha, and Raquel P. F. Guiné. 2023. "Characterisation of Lacto-Fermented Cricket (Acheta domesticus) Flour and Its Influence on the Quality Parameters and Acrylamide Formation in Wheat Biscuits" Fermentation 9, no. 2: 153. https://doi.org/10.3390/fermentation9020153
APA StyleBartkiene, E., Zokaityte, E., Kentra, E., Starkute, V., Klupsaite, D., Mockus, E., Zokaityte, G., Cernauskas, D., Rocha, J. M., & Guiné, R. P. F. (2023). Characterisation of Lacto-Fermented Cricket (Acheta domesticus) Flour and Its Influence on the Quality Parameters and Acrylamide Formation in Wheat Biscuits. Fermentation, 9(2), 153. https://doi.org/10.3390/fermentation9020153