Chemical and Aromatic Changes during Fermentation of Kombucha Beverages Produced Using Strawberry Tree (Arbutus unedo) Fruits
Abstract
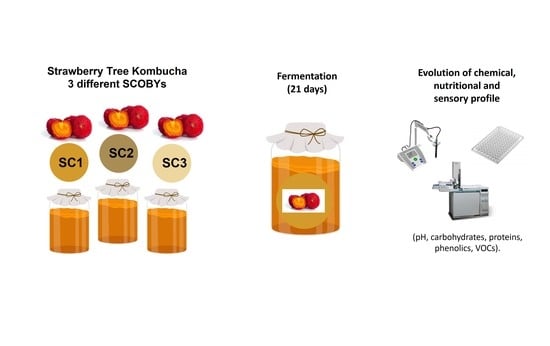
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Reagents
2.3. Microbial Analysis
2.4. Kombucha Production Using SCOBYs 1, 2, and 3
2.5. pH Measurements
2.6. Determination of Total Carbohydrates
2.7. Determination of Total Soluble Proteins
2.8. Determination of Total Phenolic Content
2.9. Analysis of Volatile Organic Compounds (VOCs) Using SPME-GC-MS
2.10. Statistical Analyses
3. Results and Discussion
3.1. Microbial Composition of the Different SCOBYs
3.2. Evolution of pH during Fermentation
3.3. Evolution of Total Carbohydrate Content during Fermentation
3.4. Evolution of Soluble Protein Content during Fermentation
3.5. Evolution of Total Phenolic Content during Fermentation
3.6. Evolution of VOCs during Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kapp, J.M.; Sumner, W. Kombucha: A Systematic Review of the Empirical Evidence of Human Health Benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Chemical Composition of Kombucha. Beverages 2022, 8, 45. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Cotter, P.D.; Souchard, J.P.; Taillandier, P.; Beaufort, S. Metabolome-Microbiome Signatures in the Fermented Beverage, Kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef] [PubMed]
- de Roos, J.; de Vuyst, L. Acetic Acid Bacteria in Fermented Foods and Beverages. Curr. Opin. Biotechnol. 2018, 49, 115–119. [Google Scholar] [CrossRef]
- da Silva Júnior, J.C.; Mafaldo, Í.M.; de Lima Brito, I.; de Magalhães Cordeiro, A.M.T. Kombucha: Formulation, Chemical Composition, and Therapeutic Potentialities. Curr. Res. Food Sci. 2022, 5, 360–365. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The Evaluation of Chemical, Antioxidant, Antimicrobial and Sensory Properties of Kombucha Tea Beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Morales, D. Biological Activities of Kombucha Beverages: The Need of Clinical Evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Recent Advances in Kombucha Tea: Microbial Consortium, Chemical Parameters, Health Implications and Biocellulose Production. Int. J. Food Microbiol. 2022, 377, 109783. [Google Scholar] [CrossRef]
- Teixeira Oliveira, J.; Machado da Costa, F.; Gonçalvez da Silva, T.; Dotto Simões, G.; dos Santos Pereira, E.; Quevedo da Costa, P.; Andreazza, R.; Cavalheiro Schenkel, P.; Pieniz, S. Green Tea and Kombucha Characterization: Phenolic Composition, Antioxidant Capacity and Enzymatic Inhibition Potential. Food Chem. 2023, 408, 135206. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Wang, H.; Jiao, S.; Wu, J.; Hou, Y.; Sun, J.; Yuan, J. Chemical Profile and Antioxidant Capacity of Kombucha Tea by the Pure Cultured Kombucha. LWT 2022, 168, 113931. [Google Scholar] [CrossRef]
- Freitas, A.; Sousa, P.; Wurlitzer, N. Alternative Raw Materials in Kombucha Production. Int. J. Gastron. Food Sci. 2022, 30, 100594. [Google Scholar] [CrossRef]
- Tenuta, M.C.; Tundis, R.; Xiao, J.; Loizzo, M.R.; Dugay, A.; Deguin, B. Arbutus Species (Ericaceae) as Source of Valuable Bioactive Products. Crit. Rev. Food Sci. Nutr. 2019, 59, 864–881. [Google Scholar] [CrossRef]
- Morales, D. Use of Strawberry Tree (Arbutus unedo) as a Source of Functional Fractions with Biological Activities. Foods 2022, 11, 3838. [Google Scholar] [CrossRef]
- Ait Lhaj, Z.; Bchitou, R.; Gaboun, F.; Abdelwahd, R.; Benabdelouahab, T.; Kabbour, M.R.; Pare, P.; Diria, G.; Bakhy, K. Moroccan Strawberry Tree (Arbutus unedo L.) Fruits: Nutritional Value and Mineral Composition. Foods 2021, 10, 2263. [Google Scholar] [CrossRef]
- Anjos, O.; Canas, S.; Gonçalves, J.C.; Caldeira, I. Development of a Spirit Drink Produced with Strawberry Tree (Arbutus unedo L.) Fruit and Honey. Beverages 2020, 6, 38. [Google Scholar] [CrossRef]
- Oliveira, I.; Guedes De Pinho, P.; Malheiro, R.; Baptista, P.; Pereira, J.A. Volatile Profile of Arbutus unedo L. Fruits through Ripening Stage. Food Chem. 2011, 128, 667–673. [Google Scholar] [CrossRef]
- Torán-Pereg, P.; del Noval, B.; Valenzuela, S.; Martinez, J.; Prado, D.; Perisé, R.; Arboleya, J.C. Microbiological and Sensory Characterization of Kombucha SCOBY for Culinary Applications. Int. J. Gastron. Food Sci. 2021, 23, 100314. [Google Scholar] [CrossRef]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Fall, A.; Daube, G.; Coton, E. Unraveling Microbial Ecology of Industrial-Scale Kombucha Fermentations by Metabarcoding and Culture-Based Methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha Beverage from Green, Black and Rooibos Teas: A Comparative Study Looking at Microbiology, Chemistry and Antioxidant Activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Tejedor-Calvo, E.; Morales, D.; García-Barreda, S.; Sánchez, S.; Venturini, M.E.; Blanco, D.; Soler-Rivas, C.; Marco, P. Effects of Gamma Irradiation on the Shelf-Life and Bioactive Compounds of Tuber Aestivum Truffles Packaged in Passive Modified Atmosphere. Int. J. Food Microbiol. 2020, 332, 108774. [Google Scholar] [CrossRef]
- de Vero, L.; Gala, E.; Gullo, M.; Solieri, L.; Landi, S.; Giudici, P. Application of Denaturing Gradient Gel Electrophoresis (DGGE) Analysis to Evaluate Acetic Acid Bacteria in Traditional Balsamic Vinegar. Food Microbiol. 2006, 23, 809–813. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R. de Kombuchas from Green and Black Teas Have Different Phenolic Profile, Which Impacts Their Antioxidant Capacities, Antibacterial and Antiproliferative Activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of Fermentation Conditions on the Production of Bioactive Compounds with Anticancer, Anti-Inflammatory and Antioxidant Properties in Kombucha Tea Extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Smiderle, F.R.; Morales, D.; Gil-Ramírez, A.; de Jesus, L.I.; Gilbert-López, B.; Iacomini, M.; Soler-Rivas, C. Evaluation of Microwave-Assisted and Pressurized Liquid Extractions to Obtain β-D-Glucans from Mushrooms. Carbohydr. Polym. 2017, 156, 165–174. [Google Scholar] [CrossRef]
- Ramírez-Anguiano, A.C.; Santoyo, S.; Reglero, G.; Soler-Rivas, C. Radical Scavenging Activities, Endogenous Oxidative Enzymes and Total Phenols in Edible Mushrooms Commonly Consumed in Europe. J. Sci. Food Agric. 2007, 87, 2272–2278. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; García-Barreda, S.; Sánchez, S.; Morales, D.; Soler-Rivas, C.; Ruiz-Rodriguez, A.; Sanz, M.Á.; Garcia, A.P.; Morte, A.; Marco, P. Supercritical CO2 Extraction Method of Aromatic Compounds from Truffles. LWT 2021, 150, 111954. [Google Scholar] [CrossRef]
- Gómez, I.; Lavega-gonzález, R.; Tejedor-calvo, E.; Pérez-Clavijo, M.; Carrasco, J. Odor Profile of Four Cultivated and Freeze-Dried Edible Mushrooms by Using Sensory Panel, Electronic Nose and GC.MS. Foods 2022, 8, 953. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. R Package, Version 1.0. 5.999. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Core Team: New York, NY, USA, 2017.
- Ferremi Leali, N.; Binati, R.L.; Martelli, F.; Gatto, V.; Luzzini, G.; Salini, A.; Slaghenaufi, D.; Fusco, S.; Ugliano, M.; Torriani, S.; et al. Reconstruction of Simplified Microbial Consortia to Modulate Sensory Quality of Kombucha Tea. Foods 2022, 11, 3045. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Qi, L.; Liang, H.; Lin, X.; Li, S.; Yu, C.; Ji, C. Effect of Synthetic Microbial Community on Nutraceutical and Sensory Qualities of Kombucha. Int. J. Food Sci. Technol. 2020, 55, 3327–3333. [Google Scholar] [CrossRef]
- May, A.; Narayanan, S.; Alcock, J.; Varsani, A.; Maley, C.; Aktipis, A. Kombucha: A Novel Model System for Cooperation and Conflict in a Complex Multi-Species Microbial Ecosystem. PeerJ 2019, 7, e7565. [Google Scholar] [CrossRef] [PubMed]
- Andreson, M.; Kazantseva, J.; Kuldjärv, R.; Malv, E.; Vaikma, H.; Kaleda, A.; Kütt, M.L.; Vilu, R. Characterisation of Chemical, Microbial and Sensory Profiles of Commercial Kombuchas. Int. J. Food Microbiol. 2022, 373, 109715. [Google Scholar] [CrossRef]
- Kaewkod, T.; Bovonsombut, S.; Tragoolpua, Y. Efficacy of Kombucha Obtained from Green, Oolongand Black Teas on Inhibition of Pathogenic Bacteria, Antioxidation, and Toxicity on Colorectal Cancer Cell Line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of Kombucha Consortium to Transform Soy Whey into a Novel Functional Beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Lin, L.; Li, C.; Jin, C.; Peng, Y.; Hashem, K.M.; Macgregor, G.A.; He, F.J.; Wang, H. Sugar and Energy Content of Carbonated Sugar-Sweetened Beverages in Haidian District, Beijing: A Cross-Sectional Study. BMJ Open 2018, 8, e022048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.F.; Xu, Y.Q.; Yuan, H.B.; Luo, L.X.; Qian, X.J. Cream Formation and Main Chemical Components of Green Tea Infusions Processed from Different Parts of New Shoots. Food Chem. 2009, 114, 665–670. [Google Scholar] [CrossRef]
- Kaashyap, M.; Cohen, M.; Mantri, N. Microbial Diversity and Characteristics of Kombucha as Revealed by Metagenomic and Physicochemical Analysis. Nutrients 2021, 13, 4446. [Google Scholar] [CrossRef]
- Zitouni, H.; Hssaini, L.; Ouaabou, R.; Viuda-Martos, M.; Hernández, F.; Ercisli, S.; Ennahli, S.; Messaoudi, Z.; Hanine, H. Exploring Antioxidant Activity, Organic Acid, and Phenolic Composition in Strawberry Tree Fruits (Arbutus unedo L.) Growing in Morocco. Plants 2020, 9, 1677. [Google Scholar] [CrossRef]
- Žlabur, J.Š.; Bogdanović, S.; Voća, S.; Babojelić, M.S. Biological Potential of Fruit and Leaves of Strawberry Tree (Arbutus unedo L.) from Croatia. Molecules 2020, 25, 5102. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Sui, Y.C.; Wu, H.W.; Zhou, C.B.; Hu, X.C.; Zhang, J. Flavour Chemical Dynamics during Fermentation of Kombucha Tea. Emir. J. Food Agric. 2018, 30, 732–741. [Google Scholar] [CrossRef]







| Nº | Name | CAS | Chemical Group | Odor Description * | RT | RI Exp | RI Nist | Mass (m/z) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Acetaldehyde | 75-07-0 | Aldehyde | Pungent, ether | 1.467 | <500 | No data | 44 | 43 | 42 |
| 2 | Ethanol | 64-17-5 | Alcohol | Sweet | 1.568 | <500 | 450 | 45 | 46 | 43 |
| 3 | 2-propanone | 67-64-1 | Ketone | Solvent | 1.64 | <500 | 500 | 43 | 58 | 42 |
| 4 | Hexane | 110-54-3 | Alkane | Alkane | 2.014 | Standard | - | 57 | 86 | 56 |
| 5 | Ethyl-acetate | 141-78-6 | Acetates | Pineapple | 2.108 | 612 | 612 | 43 | 80 | 70 |
| 6 | 2-methyl-1-propanol | 78-83-1 | Alcohol | Ethereal, whisky | 2.107 | 612 | 626 | 43 | 74 | 55 |
| 7 | Acetic-acid | 64-19-7 | Acid | Sour | 2.584 | 660 | 660 | 43 | 60 | 45 |
| 8 | benzene | 71-43-2 | Hydrocarbons aromatics | Sweet | 2.676 | 679 | 657 | 78 | 51 | 50 |
| 9 | Acetoin | 513-86-0 | Ketone | Butter, cream | 3.297 | 720 | 720 | 45 | 43 | 88 |
| 10 | 3-methyl-1-butanol | 123-51-3 | Alcohol | Whisky, malt, burnt | 3.47 | 729 | 730 | 55 | 70 | 57 |
| 11 | 2-methyl-1-butanol | 137-32-6 | Alcohol | Wine, onion | 3.506 | 730 | 733 | 57 | 70 | 56 |
| 12 | 2cyclopentene-1-4dione | 930-60-9 | Ketone | - | 8.01 | 884 | 880 | 96 | 42 | 54 |
| 13 | Decane | 124-18-5 | Alkane | Alkane | 12.247 | Standard | - | 57 | 43 | 41 |
| 14 | 2-ethyl-1-hexanol | 104-76-7 | Alcohol | Rose, green | 13.248 | 1029 | 1031 | 57 | 41 | 43 |
| 15 | Benzyl-alcohol | 100-51-6 | Alcohol | Rose, phenolic | 13.544 | 1038 | 1037 | 79 | 108 | 77 |
| 16 | Undecane | 1120-21-4 | Alkane | Alkane | 15.648 | Standard | - | 57 | 43 | 71 |
| 17 | Benzene-ethanol | 60-12-8 | Alcohol | Rose | 16.131 | 1115 | 1116 | 91 | 122 | 65 |
| 18 | benzaldehyde-4-ethyl | 4748-78-1 | Aldehyde | Sweet | 19.193 | 1214 | 1163 | 134 | 133 | 105 |
| 19 | Nonanoic-acid | 112-05-0 | Acid | Green, fat | 20.757 | 1268 | 1264 | 60 | 73 | 57 |
| 20 | 2,4-Di-tert-butylphenol | 96-76-4 | Hydrocarbons aromatics | Phenolic | 27.061 | 1514 | 1518 | 191 | 57 | 206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejedor-Calvo, E.; Morales, D. Chemical and Aromatic Changes during Fermentation of Kombucha Beverages Produced Using Strawberry Tree (Arbutus unedo) Fruits. Fermentation 2023, 9, 326. https://doi.org/10.3390/fermentation9040326
Tejedor-Calvo E, Morales D. Chemical and Aromatic Changes during Fermentation of Kombucha Beverages Produced Using Strawberry Tree (Arbutus unedo) Fruits. Fermentation. 2023; 9(4):326. https://doi.org/10.3390/fermentation9040326
Chicago/Turabian StyleTejedor-Calvo, Eva, and Diego Morales. 2023. "Chemical and Aromatic Changes during Fermentation of Kombucha Beverages Produced Using Strawberry Tree (Arbutus unedo) Fruits" Fermentation 9, no. 4: 326. https://doi.org/10.3390/fermentation9040326
APA StyleTejedor-Calvo, E., & Morales, D. (2023). Chemical and Aromatic Changes during Fermentation of Kombucha Beverages Produced Using Strawberry Tree (Arbutus unedo) Fruits. Fermentation, 9(4), 326. https://doi.org/10.3390/fermentation9040326