1. Introduction
Bananas are large herbaceous monocotyledons grown mainly as food crops (cooking bananas and plantains, <70%) and to a lesser extent as cash crops (dessert bananas and some plantains, >30%) in West and Central Africa [
1,
2]. They play an important role in agriculture in most tropical countries, where they are a staple food and an important component of food security, as well as a major source of income and services for the various actors in the sector [
3,
4]. Plantain, which accounts for an estimated 73% of production, is grown and consumed in West and Central Africa (WCA), although increasing quantities are exported to the USA and Europe [
5]. This cooking banana, known as plantain, has not been the subject of as much research and experimentation as other banana groups, despite the fact that production varies greatly from season to season due to climatic hazards and pests, among other things [
6].
The inflorescence of the banana plant, commonly known as the “banana bunch”, has the peculiarity of having female and male flowers on the same floral axis. The female flowers form first and then produce fruit. They are followed by the “male bud” which, at the time of anthesis, releases male flowers (which usually fall shortly after flowering) every day, sometimes even after the bunch has fully matured [
7]. In wild bananas, the ripe fruits are filled with black, very hard seeds, whereas in cultivated or edible bananas, the ovaries of the female flowers fill with pulp to form the edible fruit, without pollination or seed formation [
8]. Female sterility is very high, if not complete, in many cultivated clones.
Cultivated bananas are made up of a very wide range of genetic groups, all derived from two wild species,
Musa acuminata and
Musa Balbisiana. While wild banana plants are all diploid (2n = 2x = 22), cultivated varieties can also be diploid (particularly in the centre of origin), but are usually triploid (2n = 3x = 33) and much more rarely tetraploid (2n = 4x = 44) [
9]. To date, no fewer than 1000 edible varieties have been identified, divided into six genomic groups of unequal importance: AA, AAA, AB, AAB, ABB, and ABBB. All this great diversity is conserved either in vitro in the laboratory or under real conditions in genetic resource collections around the world [
10,
11,
12]. These collections are generally part of genetic improvement programmes that routinely use them for varietal creation [
13].
Varietal creation by genealogical selection in banana is based on exploiting the residual fertility of triploid cultivars (AAA or AAB-3x) by crossing them with seminiferous or parthenocarpic diploid parents (AA-2x) [
12] to produce improved dessert or cooking tetraploid hybrids. Usually, in selected tetraploids, the characteristics of the female parent are “added” to those of the male parent (with little change) [
14,
15]. In practice, tetraploid hybrids are often phenotypically close to the female parent. This suggests the transfer of a highly heterozygous maternal genome via apospory or non-reduced gametes (restitution of the first or second division of meiosis). This strategy offers little genetic variability due to the reduced number of progenitors, and relies on the production of fixed non-reduced female gametes (or gametes with low variability) due to possible recombination. It was the first to be implemented in bananas by British breeders [
16]. It has been adopted by numerous improvement programmes such as the “Institute of Tropical Agriculture (IITA)”, the “Centre Africain de Recherches sur Bananiers et Plantains (CARBAP)” at Cameroon, and the “Fundación Hondureña de Investigacíon Agrícola (FHIA)” at Honduras. Based on this strategy, these programmes have developed numerous hybrids with resistance to black leaf streak and other pests, in addition to their agronomic performance [
17,
18]. The development of these hybrids has been a major achievement in plant breeding and, in the specific case of plantain-type allotetraploids, has led to widespread distribution in Central and West Africa [
19]. The progress made by the breeders would have been greater if the problem of activating the Banana Streak Virus (BSV) sequences integrated into the B genome had not limited the potential of this breeding strategy [
17,
20]. In addition, these tetraploids have undesirable agronomic characteristics (large size, loss of vigour, drooping foliage, long fruit-filling time) and poor post-harvest fruit quality (fruit sometimes containing seeds and loss of flesh firmness), which reduces their market acceptability [
16,
21].
More recently, because of the many defects found in tetraploid plantain hybrids, particularly the integration of BSV into their genome, breeding strategies have been redirected towards a new 4x/2x breeding scheme [
21]. The ultimate goal is to produce secondary triploids from which the desired improved varieties are selected. However, the recombinations that occur during meiosis in tetraploids “break” the value of the genetic combination of the original highly heterozygous triploid parent (the result of long human selection) and they generate enormous variability in gametic populations [
14] requiring selection on very large progenies in the field. This last objective is difficult to achieve because of the low seed rate obtained by crossing banana plants. In fact, the seed fertility of banana plants is low, often in the order of 1 to 5 seeds per 100 fruits, whereas each fruit can contain at least 300 ovules [
22].
Hybridisation and seed production are the main preoccupation of any varietal creation effort, and knowledge of these processes is unfortunately limited in the case of bananas [
23]. In order to increase the efficiency of banana breeding, it is advisable to increase the quantity of seeds used per cross. As far as the genetic improvement of CARBAP is concerned, the seed production achieved by the new strategic direction of 4x/2x variety creation remains low and limits the breeders’ ability to increase the number of seeds crossed to obtain the best possible selection of desirable varieties. The aim of this study is to increase seed production in controlled crossbreeding in banana plants on both the female and male sides. In particular, it will look at how more seed can be obtained by using tetraploid hybrids as male parents, and study the value of their progeny.
2. Materials and Methods
2.1. Experimental Sites
Hybridisation data were recorded at two stations with contrasting climatic characteristics: Njombe and Ekona. These two stations are located in the agro-ecological zone known as the monomodal rainfall zone, characterised by a tropical monsoon climate and a coastal trade wind climate (Am) according to the world map of the Köppen-Geiger climate classification. The Njombe station (4°35′ N, 9°39′ E) is located at an altitude of 80m. It houses the genetic resources collection of the African Centre for Banana and Plantain Research (CARBAP). The Ekona station (4°14′00″ N, 9°20′04″ E) is located at an altitude of 400m. It is the site of the hybridisation plots containing the main parent lines used in the genetic improvement programme of the CARBAP. At each trial site, there was at least one line of 5 banana plants per genitor used.
2.2. Plant Material
The main plant material for this study is made up of allotetraploid banana hybrids, all of which are of the cooking type of banana in the green phase prior to ripening (
Table 1). Diploid monospecific Acuminata bananas have also been used in crosses with allotetraploid bananas. These were the seminiferous species Calcutta 4 (subsp. burmanicoïdes), THA 018, Pahang (subsp. malaccensis), Zebrina (subsp. zebrina), and Banksii 0623 (subsp. banksii), as well as improved parthenocarpic hybrids.
2.3. Analysis of Hybridisation Results from Njombe
The database of hybridisations carried out between 2010 and 2016 at the Njombe station was analysed in order to assess the level of seed fertility of the tetraploid progenitors traditionally used as female parents in CARBAP’s genetic improvement programme.
2.4. Evaluation of the Optimal Seed Fertility of Allotetraploids
Some hybridisations carried out in 2014 at the Ekona station, using the parthenocarpic diploid CRBP 436 as the male progenitor, were also analysed on the basis of seed rates in crosses with tetraploid female progenitors. The results of these hybridisations provided the basis for the following study.
The following study therefore consisted of a performance test to assess the real seed production potential of all the tetraploid progenitors under conditions of good pollen fertility. These accessions were crossed with pollen from the Calcutta 4 clone. The unit of comparison was the number of seeds obtained from the cross, expressed as an average per hybridised bunch and then expressed per hybridised fruit.
2.5. Characterisation of the Maturity of the Embryo Sac by DIC
All cytological and reproductive biology studies were carried out between 2018 and 2021 on samples collected exclusively in Njombe. The embryo sacs were studied using the method described by [
2]. The embryo sacs were observed exclusively in tetraploid hybrids with the seminiferous Calcutta 4 accession as a female fertility control. The rate of morphological maturity of the embryo sacs was the main unit of characterisation. This parameter represents the ratio between the number of embryo sacs that have reached full maturity (C stage) and the total number of embryo sacs corresponding to all the stages observed. The determination of the morphological maturity of the embryo sacs allowed the number of mature ovules per fruit to be estimated using the formula:
2.6. Cytological Characterisation of Pollen
The study of macrogametophytes at the mitosis II stage of meiosis, as well as at anthesis, was carried out according to the method described by [
25]. This study was conducted on allotetraploids. Calcutta 4 was used as a pollen fertility control.
In practice, the male buds harvested in the morning were taken immediately to the laboratory. Samples were taken between 07:00 and 09:00 in the morning for each of the clones studied. Pollen viability was assessed on pollen collected from flower buds located on the bract below the one uncovered at anthesis. The observations were repeated 9 times. Pollen viability was assessed under a light microscope at 64× magnification after staining with Alexander and mounting between slide and coverslip. Viability was expressed as a percentage of the number of viable pollen in relation to the total number of pollen observed.
The harvested male bud was then gradually stripped (by manually opening the spathes) down to a lower flowering stage. The desired flowering stage is that corresponding to the male flowers containing the meiospores emerging from mitosis II of meiosis. This stage is identified by progressive observation of the microspores extracted from the male flowers after the discovery of the bracts below the anthesis bract. This stage was detected using acetic carmine. The observations were repeated 9 times for each clone, under a light microscope at 640× magnification, after mounting between slide and coverslip.
2.7. Characterisation of the Fertility of Allotetraploids’ Pollen to Produce Seeds by Cross-Pollination
Pollen from allotetraploid banana hybrids was collected at anthesis to pollinate seminiferous diploid bananas. The hybridised bunches were harvested at maturity to assess the quantity of seeds produced by the cross combination.
2.8. Phenotypic Characterisation of the Progeny of the Banksii 0623 (♀) X FHIA 21 (♂) Cross
One of the crosses made in the previous phase (Banksii 0623 X FHIA 21) was followed in the field to assess the value of the progeny. This cross had to be castrated (removal of the anthers at the top of the female flowers) on the day before the ovaries of Banksii became receptive, due to its hermaphroditic status.
The progeny of the Banksii 0623 (♀) X FHIA 21 (♂) cross were evaluated phenotypically. The hybrids obtained after crossing, embryo rescue, weaning, and field transfer were evaluated according to the traits developed by [
26]. Pulp firmness, also known as pulp hardness, was measured on unripe fruit using a penetrometer. The values obtained were used to classify the fruit into 3 categories (very soft, soft and firm). From an agronomic point of view, the plot received minimal maintenance (weeding and support for bunch-bearing plants), with no inputs and no irrigation during the dry season. The aim was to stress the plants to induce the expression of activatable endogenous viral sequences.
2.9. Data Processing and Analysis
Descriptive analyses of hybridisation data were performed using R software version 4.2.2 [
27]. The data from the cytological characterisation of the pollen were subjected to an analysis of variance. In the case of a significant difference after the analysis of variance, a multiple comparison of means was performed at the 5% threshold.
3. Results
3.1. Analysis of Hybridisation Results from Njombe
Overall, this study records the results of a large number of hybridisations carried out at Njombe (501 crosses). The large number of hybridisations also reflects the repeated use of a wide variety of diploid parthenocarpic male genitors (
Table 2).
In terms of total seed production, CARBAP 832 clearly stands out with the best result, followed by its inbred counterparts CARBAP 838 and 969. Its production potential is significant, especially in one case where 81 seeds were obtained in a cross (CARBAP 832 × A26). However, to obtain large quantities of seeds, it will be necessary to make several crosses.
It is therefore possible to obtain seeds from crosses with these genitors, although sometimes there are crosses without seeds. This variation in the number of seeds in the crosses is quite well reflected in the averages obtained, where the differences that were once apparent between the tetraploid female genitors CARBAP 838, 969, and CRBP 39 are cancelled out and even approach the average value for CARBAP 832 (10 seeds).
However, FHIA 21 is an exception with a low number of crosses due to its low success rate. In fact, FHIA 21 has the lowest average seed production (3 seeds), although the variation in seed quantity between the extremes is significant for all tetraploid lines.
A divergence in bunch maturation was observed depending on the tetraploid female genitors. They are divided into two groups, the first consisting of CRBP 39 and FHIA 21 with short maturation (Interval between Flowering and Harvest—IFC < 100 days), and the second consisting of CARBAP 832, 838, and 969, characterised by prolonged maturation (IFC > 100 days). The seed results follow the same trend: one group of tetraploid female genitors in which more than half of the bunches contained seeds (CARBAP 832, 838 and 969), and another group of female tetraploid genitors in which at most two out of five bunches contained seeds (CRBP 39 and FHIA 21).
3.2. Optimum Seed Production Performance of Allotetraploid Hybrids
At Ekona, pollination with CRBP 436 (CARBAP’s improved diploid) significantly increased seed production of these tetraploid hybrids (
Table 3). Although the variation in the number of seeds per cross is always significant, the maximum values obtained are 514, 276, and 167 seeds in a single cross with CARBAP 832, 838, and 969 respectively. Only FHIA 21 has consistently low numbers (four seeds at best). Success rates in crosses are also higher than 80% for the majority, with the exception of FHIA 21 with 67%. These observations do not allow us to conclude whether the significant increase in seed quantity is due to the progenitor CRBP 436 or to environmental conditions more favourable to fruit at the Ekona station. These results call into question the true seed production capacity of tetraploid genitors.
In Njombe, these allotetraploid genitors were therefore studied to assess their maximum seed production potential when pollinated by seminiferous banana plants, which are characterised by high pollen fertility (
Table 4). The quantities of seed-bearing bunches per cross (80–100%) are significantly higher than previous results with the same tetraploid female breeding lines. The highest averages were obtained with CRBP 39 (304 and 215 seeds), followed by CARBAP 832 (162 seeds) and finally CARBAP 969 (101 seeds). Although the number of seeds is very variable for the same parental combination, the values obtained are generally higher than 100 seeds per cross for the clones CRBP 39, CARBAP 832, 838, and 969.
Clone FHIA 21 is another exception, with a maximum of only 41 and 75 seeds in crosses with Calcutta 4 and Zebrina, respectively. These values are among the lowest of all.
However, the average quantitative estimates of seeds per fruit suggest that the seed fertility of these genitors is low. In the best case, the fruits of the most fertile (CRBP 39 x Calcutta 4) carry 5 seeds, and in cases of total failure, all fruits of the hybridised bunch contain no seeds (FHIA 21 x Banksii 623).
3.3. Morphological Maturity of Allotetraploid Embryo Sacs
The low number of seeds per fruit in the tetraploid genitors, despite pollination with seminiferous banana plants known for their good male fertility, prompted an assessment of the presence of female gametes (embryo sacs) in the ovary, as well as their maturity and proportion at anthesis. Calcutta 4 was used as the seminiferous reference genotype for this study.
The proportion of aborted sacs in the seminiferous monospecific AA clone Calcutta 4 is surprisingly high (almost 50%), despite the fact that this clone does not in principle present any reproductive barriers, particularly at the meiotic level (
Table 5). In contrast, the tetraploidy and the interspecific
acuminata/
balbisiana constitution of the AAAB hybrids did not prevent the formation of functional embryo sacs at fertilisation. Two patterns of embryo sac development were observed among the clones studied. In the first group, the embryo sacs at anthesis seem to be blocked and aborted at stages A2 and B1, while those that have reached stages B2 and B3 seem to develop in some cases towards full maturity (stage C) in the days following anthesis (Calcutta 4 and CARBAP 969). In the second group (CRBP 39, FHIA 21), embryo sac maturation appears to continue in the days following anthesis, whatever the stage.
Overall, the clones studied contained a significant proportion of mature, potentially fertilisable embryo sacs, ranging from 29% to 57% in the tetraploids. More than half of the embryo sacs observed 2 days after anthesis were morphologically mature in CARBAP 969 (53.33%) and FHIA 21 (57.56%). On the other hand, CRBP 39 had the lowest proportion of morphologically mature embryo sacs both at anthesis and 2 days later. This contradicts the previously observed logic regarding the seed production capacity of CRBP 39.
Evaluation of the number of eggs per ovary, which varied from 134 (Calcutta 4 clone) to 296 (FHIA 21 clone), allowed us to estimate the number of potentially fertilisable sacs (at maturity, stage C) independently of any other source of sterility. Except for clone Calcutta 4, where the number of mature ovules and the number of seeds per fruit are of about the same order (see Table 7, Calcutta 4 x THA018 cross), this estimate shows a much higher number of potentially fertilisable ovules for tetraploid hybrids than the number of seeds usually obtained by pollination with seminiferous clones, in an average ratio of around 1 to 50 (see
Table 4).
3.4. Cytological Characterisation of Pollen from Tetraploid Clones
The three main configurations of tetrads observed (linear, tetrahedral, and spherical) are characteristic of normal meiosis in banana (
Figure 1). On the other hand, we also observed products of meiotic dysfunction, in particular dyads and triads in non-negligible proportions, which are the result of chromosome restitution in the first or second mitotic division. Finally, we also observed polyads, indicating the presence of lagging chromosomes and, consequently, unbalanced chromosome distributions at the end of the two mitotic divisions. All these sporad patterns were observed, but in varying proportions, in allotetraploid hybrids and also in the Calcutta 4 clone, the pollen fertility reference for this study.
The results obtained for tetraploids illustrate a predominantly normal pollen meiosis with a variable proportion of tetrads depending on the allotetraploid hybrid (76 to 87% proportion of normal sporads—
Table 6). The proportion of failed meiotic divisions was very low, generally less than 10%, with the exception of the proportion of dyads observed in CRBP 39 and FHIA 21 (13%). These proportions are even lower for polyads. These normal meiotic sequences are not significantly different from those observed in the Calcutta 4 clone, despite some statistical differences.
These observations on sporads are reflected at anthesis by an estimated pollen viability rate of over 70% (
Table 6), with the pollen viability of allotetraploid hybrids remaining significantly lower than that of the Calcutta 4 control.
Finally, it should be noted that the number of viable pollen grains per anther in allotetraploid hybrids is on average very high and sometimes (statistically) close to the Calcutta 4 value (
Table 6). The hybrids CARBAP 832, CARBAP 969 and, to a lesser extent, CARBAP 838 have values close to the seminiferous reference, while CRBP 39 and FHIA 21 have significantly lower values.
These two cytological characteristics of allotetraploids’ pollen (high pollen viability and a large number of viable pollen grains per anther) suggest that these clones could be used as pollinators for banana breeding.
3.5. Biological Performance of Pollen from Tetraploid Clones
It is possible to obtain more seeds in crosses by using the tetraploid pollen population in a single cross, as shown by the averages obtained (
Table 7). The values range from an average of 460 seeds for CARBAP 969, through 661 seeds for CARBAP 832, up to 2930 seeds for FHIA21. However, the optimal gametic fertility potential of Calcutta 4 (more than 3000 seeds in a cross with THA 018) is almost never achieved with pollen from tetraploid genitors. In fact, their pollen efficiency is generally between 3% (pollen from CRBP 39) and 17% (pollen from CARBAP 832) of Calcutta’s seed potential.
The quantities of seeds per bunch are in some cases lower when comparing the average seed production in reciprocal crosses, as illustrated by the combinations between Calcutta and Pahang in crosses with CRBP 39 pollen (103 versus 304 seeds per bunch and 19 versus 50 seeds per bunch, respectively). Surprisingly, the quantities of seeds produced do not reflect the cytological performance of their pollen (number of pollen and pollen viability).
In practical terms, seed production was significantly improved using FHIA 21 as the male parent compared with results using the same clone as the female parent. It is shown here that seed rates in crosses with FHIA 21 as the male parent vary greatly between replicates of the same cross, and even more so when the seminiferous female parent is changed (contrast between Zebrina X FHIA 21 and other crosses). This instability in seed production is also observed with the pollen of CRBP 39, which combined with Calcutta 4 gives between 0–205 seeds, and much less with Zebrina, where we get between 0–63 seeds, despite the fact that we are working in a context of good gametic fertility (see
Table 4 and
Table 7).
Overall, exploiting the male fertility of tetraploid genitors in crosses with seminiferous clones results in a non-significant improvement in the amount of seeds per hybridised fruit. However, this small, substantial improvement in seed production in crosses is not systematic and turns out to be genitor specific.
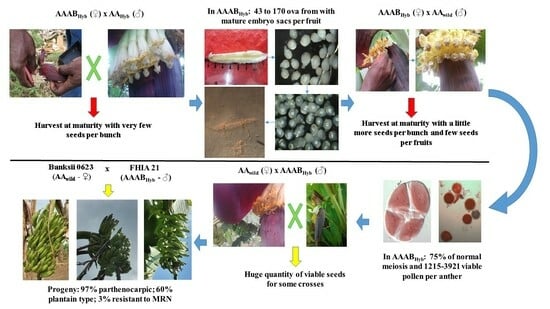
3.6. Strategy to Overcome Sterility by Reversing the Crossing Pattern with Banksii
The progeny of the Banksii 0623 X FHIA 21 cross was characterised in the field at the phenotypic level only, in order to assess its value in an approach aimed at creating cooking banana varieties. This conventional scheme Banksii 623 (♀) X FHIA 21 (♂) produces more than 400 seeds in a single cross, while no seeds were obtained by reciprocal crossing (
Table 8).
In in vitro culture, the embryos, most of which were normal, germinated at a rate close to 100%, with the exception of minimal losses due to infections in petri dishes.
Major losses of plant material (57.71%) were recorded during the handling of plants at the weaning stage and transfer to the field. It should be noted that all plants showing signs of BSV were completely destemmed and destroyed. Other losses were linked to the stunted appearance of the hybrids, which were unable to survive the long, intense dry season. In the end, only 74 plants remained, of which 62 were characterised in terms of vegetative and floral characteristics.
Vegetative characteristics in the hybrid population were highly variable, both in terms of plant height (14% coefficient of variation) and circumference (16% coefficient of variation). Moreover, the mean values obtained, 278.81 cm and 50.28 cm for height and circumference respectively, are higher than the maximum values measured in the parents (
Table 9).
The harvest characteristics also show a wide variation in certain parameters, particularly in the weight of the bunches and the circumference of the fruit (38.52% and 45.24% respectively), with results sometimes higher than the average of their ancestor. On the other hand, the average length of the fruit did not vary as much as previously observed (29.62%), with values ranging from 87 to 429 mm. The number of hands in the bunches studied (7 on average) showed a similar trend to the number of fruits per hand, with values of 7 hands for an average of 12 fruits/hand.
Half of the progeny of this cross are triploid hybrids, most likely the result of normal gamete distribution events in the progeny. This may not be the case for the remaining two quarters, which are almost equally divided between tetraploids and aneuploids. The majority of these progeny are 97% parthenocarpic hybrids (
Figure 2A), more than half of which are classified as “plantain type” based on the colour of their flesh (ivory;
Figure 2B) and their firmness when green.
Finally, 14 plants (23%) proved to be tolerant to MRN, depending on the symptoms observed and the number of living leaves when the bunches were harvested.
4. Discussion
The tetraploid progenitors traditionally used as female parents all produce seeds when pollinated by diploid parthenocarpic banana plants. This crossing scheme, widely used by CARBAP, generally proves to be the most productive compared to crossing with parthenocarpic male progenitors at the triploid and tetraploid levels [
28]. However, our results show a very large variation in seed production, to the point where sometimes no seeds are obtained after crossing. In addition, the fertility rates at the bunch level (29–80%) mean that the number of crosses needs to be multiplied in order to increase the quantity of seeds in the event of fertility. However, these seed quantities are very low when the totals are compared with the averages in the crosses. The tetraploid FHIA 21 is a perfect example, with a maximum production of 6 seeds and an average of 3 seeds per cross. Its history as a female parent in the CARBAP genetic improvement programme shows that it is very low in fertility, even when conditions appear favourable (FHIA 21 X CRBP 436 at Ekona). In contrast, the potential of other genitors under different conditions improved, but never exceeded an average of 5 seeds per fruit, even when pollinated with seminiferous banana plants known for their good gametic fertility. This quantity of seeds produced by cross-pollination is much lower than what would have been obtained if all the ovules present in the ovaries had been fertilised at anthesis [
2]. There are three main mechanisms responsible for the absence of seeds: mechanisms that inhibit the production of embryo sags; mechanisms that prevent the fertilisation of ovules; and finally, mechanisms that inhibit or abort the development of embryos as parthenocarpy [
29]. In the edible banana clones it has been observed that sterility is due to failure to form embryo sacs [
30].
The morphological maturity of the embryo sacs of a few tetraploid progenitors was therefore characterised throughout the period of ovarian receptivity. Observations in allotetraploid hybrids showed large numbers of ovules with morphologically mature embryo sacs, but very few seeds. There are between 40 and 170 potentially fertile ovules, but in the specific case of FHIA 21, for example, at most 2 seeds are produced per fruit. On the other hand, in Calcutta 4, a seminiferous variety, the maturity of the embryo sacs, although far from exceptional, is close to the quantity of seeds produced in crosses (ratio 1 seed/2 mature ovules) in a context of good pollen fertility (seminiferous banana pollen). Our results show that the characterisation of the maturity of the embryo sacs, in addition to all the other sources of sterility associated with the domestication of banana plants, makes it possible to assess the fertility rate of the fruit. Consequently, the low amounts of seeds in crosses with edible varieties are due to other factors independent of the presence of mature embryo sacs. The study conducted by [
2] goes in the same direction, asserting that it is possible to produce more than a hundred times the number of seeds actually obtained, if the presence of normal embryo sacs were the only condition. This raises the question of the viability of the embryo sacs and the probable abortion of the ovules after fertilisation. It would seem that the arrangement of embryo sacs at anthesis in the ovules, their distribution in the ovary, and above all the degeneration of its nuclei, would considerably affect the viability of the embryo sacs [
22,
31], thus contributing to the sterility of the female organs. Nevertheless, our study highlights the fact that the failure of seed production (less than 7% of mature ovules fertilised) compared with theoretical expectations (more than 57% of mature ovules available) is partly due to the absence of karyogamy at a stage prior to fertilisation, but also to seed abortion after fertilisation [
2].
The tetraploid genitors are the result of human selection over many years, not for the fertility of their seeds, but rather for the improvement of their agronomic performance [
17,
21,
32]. This human selection would therefore have led to the accumulation of numerous factors responsible not only for gametic sterility, but also for the inhibition of fertilisation, such as the necrosis observed in the distal part of the ovary one day after anthesis [
33], and obstruction of the pistillary canal by a bulge of tissue at the junction of the pistil and the ovary, preventing the pollen tubes from progressing to the embryo sacs [
29,
31]. Their genetic nature would therefore prevent any quantitative improvement in seed production. A strategy to overcome genetic sterility barriers was therefore tested to safeguard against all sources of low or non-existent gametic fertility associated with domestication. The aim was to use tetraploid banana plants as male parents in crosses with wild seminiferous clones. Their pollen potential was assessed both cytologically and biologically. Cytologically, pollen viability rates were rather high in tetraploids (70–77%) and not far from the value observed in the Calcutta 4 pollen fertility control (94%). The same applies to the proportions of meiospores with a normal configuration, whose values, following the previous parameter, are much closer to each other. These good theoretical efficiency references motivated their use in crosses with naturally fertile wild seminiferous clones. The results of hybridisation show that pollen efficiency is important. Although with FHIA 21 pollen it was possible to achieve a high value in a single cross (2930 seeds), it remains true that overall seed rates have not really been improved. In fact, they have never reached the levels observed under conditions of good fertility with wild seminiferous clones (over 3000 seeds with an average of 31 seeds per fruit). Is this mitigated performance of tetraploid pollen in crosses the result of a poorly executed hybridisation technique (perfect synchronisation between stigma receptivity and pollination)? This question, for which no solution has been proposed in this study, raises the hypothesis of a high proportion of pollen with different ploidy levels (polysporia), which is very common in allotetraploids, and which could possibly have a selective influence on the germination of pollen on stigmas [
34,
35]. In addition, the allotetraploid nature of these hybrids could also have a significant effect on the proportion of pollen fertile at anthesis, greatly reducing their performance when used in crosses [
20,
36].
In view of the improvement potential observed by using FHIA 21 pollen in crosses, the next step was to assess the value of the progeny by focusing the strategy on the creation of cooking banana varieties. To this end, the cross Banksii 0623 (♀) X FHIA 21 (♂) was selected for careful phenotypic characterisation, given the cooking-type genetic background of the two accessions. The other special feature is the hermaphroditic nature of the female flowers of Banksii 0623, which gives it a more homogeneous and conserved genetic heritage [
37,
38], added to the fact that it originates from the main centre of diversification for cooking bananas (Papua New Guinea). This cross produced more than 400 seeds, a significant improvement on the zero achieved by reciprocal crossing. This represents 400 different genetic combinations in one cross, whereas reciprocal crosses require more crosses to achieve this seed rate. This improvement is of considerable benefit, as the work invested in cross-breeding can now be directed towards selecting hybrids of interest, as highlighted so well by [
39]. Phenotypic characterisation of the hybrid progeny from this cross showed that 97% were parthenocarpic, with more than 60% of the plantain type showing perfect triploidy in more than half of the cases. This implies a certain distribution of traits in the progeny of the cross and, more importantly, the formation of hybrids with an AAB genomic constitution. We can therefore assume the formation of viable and functional interspecific diploid gametes (AB). Several studies support this possibility, although they point to the imbalance of this type of gamete, which has a major impact on chromosome segregation [
20,
40]. However, in the case of resistance to Black Streak Disease (BSD), this distribution of traits from parent to offspring is less pronounced. In fact, if we start with two progenitors that are resistant to this disease, we will obtain hybrid progeny that are resistant only in the minority (3%). On the other hand, the yield characteristics sometimes show higher values than the progenitors, suggesting a heterosis effect in the progeny.
5. Conclusions
The allotetraploid banana hybrids have historically been considered the end product of genetic improvement programmes around the world. However, they have shown undesirable characteristics that have hindered their dissemination. The aim has been to transfer their genetic value to their progeny, while attempting to maintain some degree of genetic structure. They have therefore been widely used as female parents in crosses with improved parthenocarpic diploids and seminiferous diploids.
This study highlighted the low rate of seed production in this cross, which severely limits the performance in varietal creation. Cytological observations made on the female side could not explain this low gametic fertility. In fact, the quantities of seeds obtained by crossing are always much lower than, and not proportional to, the quantities of morphologically mature embryo sacs throughout the period of ovary receptivity (7% seeds/fruit vs. 57% ovules with mature embryo sacs/fruit).
Improvements in seed production have been observed using pollen from certain allotetraploid progenitors, most notably FHIA 21, by reversing conventional hybridisation patterns and avoiding any source of potential sterility associated with domestication (use of seminiferous banana plants). Exploiting the potential of FHIA 21 pollen in a crossbreeding programme focused on the creation of cooking banana varieties has been fruitful. Indeed, the Banksii 0623 (♀) X FHIA 21 (♂) combination not only led to a significant improvement in seed production, but also produced progeny with interesting vigour and phenotypic characteristics, in particular 97% parthenocarpic with over 60% plantain type and 3% resistant to MRN.
It is more important than ever to understand the causes of sterility in banana plants in order to improve breeding performance in genetic improvement programmes. However, it is possible to improve performance where seed production is low by reversing the position of the tetraploid progenitor for use in crosses with seminiferous bananas. The latter has varying degrees of potential to transfer genetic structure from parents to offspring. However, further studies (genomic, molecular, etc.) will be needed to support this new approach to the efficient genetic improvement of the cooking banana.