A Review of the Main Process-Based Approaches for Modeling N2O Emissions from Agricultural Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search Details
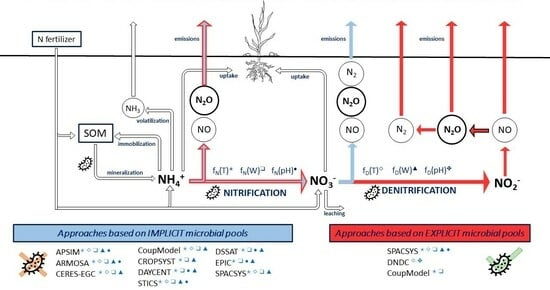
2.2. Overview of the Selected Models
| Reference | Author | Year | Model | Nit | Denit | Microbial Biomass | f(T) | f(W) | f(pH) | f(substrate) |
|---|---|---|---|---|---|---|---|---|---|---|
| [67] | Thorburn et al. | 2010 | APSIM | yes | yes | no | yes | yes | only for Nit | active carbon only for Denit |
| [56] | Perego et al. | 2013 | ARMOSA | yes | yes | no | yes | yes | only for Nit | - |
| [58] | Gabrielle et al. | 2005 | CERES-EGC | yes | yes | no | yes | yes | no | - |
| [68] | Jansson and Karlberg | 2010 | CoupModel | yes | yes | optional for both Nit and Denit | yes | yes | yes | DOC for Nit in the microbial explicit approach |
| [69] | Stockle et al. | 2012 | CROPSYST | yes | yes | only for SOC mineralization | only for Nit | yes | only for Nit | CO2 for Denit |
| [11] | Parton et al. | 2001 | DAYCENT | yes | yes | no | only for Nit | yes | only for Nit | CO2 for Denit |
| [52] | Li | 2000 | DNDC | yes | yes | yes | yes | only for Nit | yes (indirectly for Nit) | indirectly DOC for both |
| [70] | Hoogenboom et al. | 2019 | DSSAT | yes | yes | no | only for CERES-Denit and Nit | yes | only for Nit | CO2 for DAYCENT-Denit, water-extractable C for CERES-Denit |
| [62] | Sharpley and Williams | 1990 | EPIC | yes | yes | no | yes | yes | only for Nit | CO2 for Denit (Kemanian option) |
| [71] | Wu et al. | 2015 | SPACSYS | yes | yes | optional for both Nit and Denit | yes | only for Nit | yes | DOC for Nit and Denit |
| [60] | Brisson et al. | 2008 | STICS | yes | yes | no | yes | yes | only for Nit | - |
2.3. Statistical Indices for Model Evaluation
3. Results
3.1. Process-Based Models
3.1.1. Nitrification
Approaches Based on Implicit Microbial Pools
Approaches Based on Explicit Microbial Pools
3.1.2. Denitrification
Approaches Based on Implicit Microbial Pools
Approaches Based on Explicit Microbial Pools
3.1.3. Emissions
3.1.4. Environmental Factors
Soil Temperature Factor
Soil Moisture Factor
Soil Acidity Factor
Substrate Concentration Effect
3.1.5. Model Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Model Evaluation Indices
Appendix A.2. Process-Based Models Auxiliary Variables
Appendix A.3. Tables
| Model | Variable | Unit | Original Symbol | Review Symbol |
|---|---|---|---|---|
| APSIM | nitrification rate | mg N g−1 d−1 | Rnit | Rnit |
| ARMOSA | nitrification rate | kg N ha−1 d−1 | Nit_NH4 | Rnit |
| ARMOSA | nitrate amount | kg N ha−1 | NO3 | NO3 |
| ARMOSA | ammonium amount | kg N ha−1 | NH4 | NH4 |
| CERES-EGC | nitrification rate | kg N ha−1 d−1 | Ni | Rnit |
| CoupModel | nitrification rate | g N m−2 d−1 | NNH4→NO3 | Rnit |
| CoupModel | nitrifier biomass | g m−2 | NmicrN | Bnit |
| CROPSYST | nitrification rate | kg N ha−1 d−1 | NNH4→NO3 | Rnit |
| CROPSYST | ammonium amount | kg N ha−1 | NNH4 | NH4 |
| CROPSYST | nitrate amount | kg N ha−1 | NNO3 | NO3 |
| DAYCENT | nitrification rate | g N m−2 d−1 | FNO3 | Rnit |
| DAYCENT | mineralization rate | g N m−2 | Netmm | Netmm |
| DAYCENT | ammonium amount | g N m−2 | NH4 | NH4 |
| DNDC | nitrification rate | kg N ha−1 d−1 | RN | Rnit |
| DNDC | ammonium amount | kg N ha−1 | [NH4+] | NH4 |
| DNDC | nitrifier biomass | kg C ha−1 | Nitrifier | Bnit |
| DSSAT | nitrification rate | kg N ha−1 d−1 | NITRIF | Rnit |
| DSSAT | ammonium amount | kg N ha−1 | NH4 | NH4 |
| EPIC | nitrification rate | kg N ha−1 d−1 | RNIT | Rnit |
| EPIC | volatilization rate | kg N ha−1 d−1 | AVOL | Rvol |
| EPIC | ammonium amount | kg N ha−1 | NH3 | NH4 |
| EPIC | auxiliary variable | unitless | AKAV | AKAV |
| EPIC | soil temperature | °C | STMP | T |
| SPACSYS | nitrification rate (microbial explicit) | g N m−2 d−1 | nn | Rnit |
| SPACSYS | water-filled pore space | unitless | WFPS | WFPS |
| SPACSYS | nitrifier biomass | g C m−2 | Mb | Bnit |
| SPACSYS | nitrification rate | g N m−2 d−1 | Nnitri | Rnit |
| STICS | nitrification rate (layer) | kg N ha−1 cm−1 d−1 | TNITRIF | Rnit |
| STICS | nitrification rate (total) | kg N ha−1 d−1 | NITRIF | Rnit(total) |
| STICS | ammonium amount | kg N ha−1 | AMM | NH4 |
| all model | nitrification response function to soil temperature | unitless | various | fn(T) |
| all model | nitrification response function to soil moisture | unitless | various | fn(W) |
| all model | nitrification response function to soil acidity | unitless | various | fn(pH) |
| all model | nitrification response function to ammonium level | unitless | various | fn(NH4) |
| Model | Parameter | Unit | Original Symbol | Review Symbol | Default |
|---|---|---|---|---|---|
| APSIM | nitrification coefficient | mg N g−1 d−1 | Vmax | Vmax | 40 |
| APSIM | nitrification half-saturation constant | mg N g−1 | Km | Km | 90 |
| ARMOSA | nitrification coefficient | d−1 | kn | knit | 0.2 |
| ARMOSA | NO3:NH4 ratio | unitless | Nratio | rNO3/NH4 | 8 [1–15] |
| CERES-EGC | nitrification coefficient | kg N ha−1 d−1 | MNR | knit | - |
| CoupModel | pH response function coefficient | unitless | npH | kpH | 1 |
| CoupModel | nitrification coefficient | mg ha d−1 kg−1 | nmicrate | knit | 0.25 |
| CROPSYST | nitrification coefficient | d−1 | NITK | knit | 0.2 |
| CROPSYST | NO3:NH4 ratio | unitless | NITR | rNO3/NH4 | 8 [1–15] |
| DAYCENT | nitrified fraction of Netmm | d−1 | K1 | knit2 | 0.2 |
| DAYCENT | nitrified fraction of NH4+ | d−1 | Kmax | knit | 0.1 |
| DNDC | nitrification coefficient | d−1 | 0.005 | knit | 0.005 |
| EPIC | nitrified/volatilized fraction of NH3 | unitless | PARAM(64) | knit3 | [0–1] |
| SPACSYS | nitrification coefficient (microbial explicit) | d−1 | nnmax | knit | 0.004 |
| SPACSYS | nitrification coefficient | d−1 | knitri | knit | - |
| STICS | max depth for nitrification | cm | PROFHUMS | znit | 30 |
| STICS | nitrification coefficient | d−1 | FNXG | knit | 0.5 |
| STICS | N2O:total nitrification ratio | unitless | RATIONITS | rN2O/nit | - |
| Model | Variable | Unit | Original Symbol | Review Symbol |
|---|---|---|---|---|
| APSIM | denitrification rate | kg N ha−1 d−1 | Rdenit | Rdenit |
| APSIM | nitrate amount | kg N ha−1 | NO3 | NO3 |
| APSIM | active C concentration | ppm | CA | [CA] |
| ARMOSA | denitrification rate | kg N ha−1 d−1 | Denit_NO3 | Rdenit |
| ARMOSA | nitrate concentration | kg N L−1 | Conc_NO3 | [NO3] |
| CERES-EGC | denitrification rate | kg N ha−1 d−1 | PDR | Rdenit |
| CoupModel | denitrification rate | g N m−2 d−1 | NNO3→Denit | Rdenit |
| CoupModel | NxOy consumption rate | g N m−2 d−1 | NNxOy→AnNxOy | NNxOy→AnNxOy |
| CoupModel | NxOy content in the anaerobic pool | g N m−2 d−1 | AnNxOy | AnNxOy |
| CoupModel | dpot depth-adjustment coefficient | unitless | ddist | zadj |
| CROPSYST | denitrification rate | kg N ha−1 d−1 | Da | Rdenit |
| DAYCENT | denitrification rate | µg N g soil−1 d−1 | Dt | Rdenit |
| DNDC | NxOy consumption rate | kg N ha−1 d−1 | dNOx/dt | Rc,NxOy |
| DNDC | denitrifier biomass | kg N ha−1 | Denitrifier | Bdenit |
| DSSAT | denitrification rate | kg N ha−1 d−1 | DENITRIF | Rdenit |
| DSSAT | volumetric soil water content | m3 m−3 | SW | SWC |
| DSSAT | drained upper limit | m3 m−3 | DUL | DUL |
| DSSAT | soil temperature | K | ST | T |
| DSSAT | water-extractable soil C | kg C ha−1 | CW | CW |
| DSSAT | nitrate amount | kg N ha−1 | NO3 | NO3 |
| EPIC | denitrification rate | kg N ha−1 d−1 | DN | Rdenit |
| EPIC | soil weight | kg | WT | WT |
| EPIC | nitrate amount | kg N ha−1 | NO3 | NO3 |
| SPACSYS | NxOy consumption rate | kg N m−3 d−1 | dc,i | Rc,NxOy |
| SPACSYS | NxOy concentration | kg N m−3 | Ni | [NxOy] |
| SPACSYS | total NxOy concentration | kg N m−3 | Ntotal | ∑[NxOy] |
| SPACSYS | denitrification rate | g N m−2 d−1 | Ndeni | Rdenit |
| STICS | denitrification rate (total) | kg N ha−1 d−1 | NDENENG | Rdenit |
| all model | denitrification response function to soil temperature | unitless | various | fd(T) |
| all model | denitrification response function to soil moisture | unitless | various | fd(W) |
| all model | denitrification response function to soil acidity | unitless | various | fd(pH) |
| all model | denitrification response function to NxOy level | unitless/µg N g soil−1 d−1 | various | fd(NxOy) |
| all model | denitrification response function to DOC level | unitless | various | fd(DOC) |
| all model | denitrification response function to heterotrophic respiration | unitless/µg N g soil−1 d−1 | various | fd(CO2) |
| Model | Parameter | Unit | Original Symbol | Review Symbol | Default |
|---|---|---|---|---|---|
| APSIM | denitrification coefficient | Unitless | kdenit | kdenit | 0.0006 |
| ARMOSA | denitrification coefficient | kg N ha−1 d−1 | kd | kdenit | [0.04–0.2] |
| ARMOSA | denitrification half-saturation constant | mg N L−1 | Hsconst | Km | [5–15] |
| CERES-EGC | denitrification coefficient | kg N ha−1 d−1 | PDR | kdenit | - |
| CoupModel | denitrification coefficient | g N m−2 d−1 | dpot | kdenit | 0.04 |
| CROPSYST | denitrification coefficient | kg N ha−1 d−1 | Dp | kdenit | - |
| DNDC | growth yield on NxOy | kg C kg N−1 | YNOx | YNxOy | - |
| DNDC | maintenance coefficient on NxOy | kg N kg−1 h−1 | MNOx | MNxOy | - |
| DSSAT | max depth for denitrification | m | Denit_depth | zdenit | 0.3 |
| DSSAT | denitrification coefficient | d−1 | 0.0006 | kdenit | 0.0006 |
| EPIC | denitrification coefficient | Unitless | DNITMX | kdenit | 32 |
| SPACSYS | denitrification coefficient | g N m−2 d−1 | kdeni | kdenit | - |
| SPACSYS | maintenance coefficient on NxOy | g C g N−1 d−1 | MNi | MNxOy | Table A8 |
| SPACSYS | growth yield on NxOy | g C g N−1 | YNi | YNxOy | Table A8 |
| SPACSYS | growth rate on NxOy | d−1 | γgd | γgd | Table A8 |
| STICS | denitrification coefficient | kg N ha−1 d−1 | VPOTDENITS | kdenit | 16 |
| STICS | max depth for denitrification | cm | PROFDENITS | zdenit | 20 |
| Model | Variable | Unit | Original Symbol | Review Symbol |
|---|---|---|---|---|
| APSIM | nitrification N2O emission rate | kg N ha−1 d−1 | N2Onit | N2Onit |
| APSIM | denitrification N2O emission rate | kg N ha−1 d−1 | N2Odenit | N2Odenit |
| APSIM | nitrate concentration | µg N g−1 | NO3ppm | [NO3] |
| APSIM | heterotrophic respiration rate | µg C g soil−1 d−1 | CO2 | CO2,resp |
| ARMOSA | nitrification N2O emission rate | kg N ha−1 d−1 | Nit_N2O | N2Onit |
| ARMOSA | denitrification N2O emission rate | kg N ha−1 d−1 | Denit_N2O | N2Odenit |
| ARMOSA | nitrate amount | kg N ha−1 | NO3 | NO3 |
| ARMOSA | heterotrophic respiration rate | kg C ha−1 d−1 | CO2 | CO2,resp |
| ARMOSA | soil water content at saturation | m3 m−3 | SWCsat | SWCsat |
| CoupModel | nitrification NO emission rate | g N m−2 d−1 | NOnit | NOnit |
| CoupModel | emissions response function to soil temperature | unitless | fe(T) | fe(T) |
| CoupModel | emissions response function to soil moisture | unitless | fe(W) | fe(W) |
| CoupModel | emissions response function to soil acidity | unitless | fe(pH) | fe(pH) |
| CoupModel | nitrification N2O emission rate | g N m−2 d−1 | N2Onit | N2Onit |
| CROPSYST | denitrification N2O emission rate | µg N kg−1 d−1 | DN2O | N2Odenit |
| CROPSYST | N2/N2O ratio | unitless | RN2/N2O | RN2/N2O |
| CROPSYST | denitrification N2 emission rate | µg N kg−1 d−1 | DN2 | N2,denit |
| CROPSYST | emissions response function to nitrate level | unitless | fr(NO3) | fe(NO3) |
| CROPSYST | emissions response function to heterotrophic respiration | unitless | fr(CO2) | fe(CO2) |
| CROPSYST | emissions response function to soil moisture | unitless | fr(W) | fe(W) |
| DAYCENT | denitrification N2O emission rate | g N m−2 d−1 | N2Odenit | N2Odenit |
| DAYCENT | N2/N2O ratio | unitless | RN2/N2O | RN2/N2O |
| DAYCENT | DFC function | unitless | Fr(NO3/CO2) | fe(NO3/CO2) |
| DAYCENT | N2/N2O ratio response function to soil moisture | unitless | Fr(WFPS) | fe(W) |
| DAYCENT | nitrification N2O emission rate | g N m−2 d−1 | N2Onit | N2Onit |
| DAYCENT | NOx emission rate | g N m−2 d−1 | NOx | NOx,nit+denit |
| DAYCENT | NOx/N2O ratio | unitless | RNOx | RNOx/N2O |
| DAYCENT | pulse multiplier | unitless | P | P |
| DNDC | nitrification N2O emission rate | kg N ha−1 d−1 | N2ON | N2Onit |
| DNDC | denitrification N2O emitted fraction | unitless | P(N2O) | P(N2O) |
| DNDC | denitrification N2 emitted fraction | unitless | P(N2) | P(N2) |
| DSSAT | nitrification N2O emission rate | kg N ha−1 d−1 | N2Onit | N2Onit |
| DSSAT | nitrification NO emission rate | kg N ha−1 d−1 | NOnit | NOnit |
| DSSAT | NO/N2O ratio | unitless | NO_N2O_ratio | RNOx/N2O |
| DSSAT | pulse multiplier | unitless | P | P |
| DSSAT | denitrification N2O emission rate | kg N ha−1 d−1 | N2Odenit | N2Odenit |
| DSSAT | denitrification N2 emission rate | kg N ha−1 d−1 | N2 | N2,denit |
| DSSAT | N2/N2O ratio | unitless | Rn2n2o | RN2/N2O |
| DSSAT | auxiliary variable | unitless | ratio1 | ratio1 |
| DSSAT | auxiliary variable | unitless | ratio2 | ratio2 |
| DSSAT | emissions response function to nitrate level | unitless | fe(NO3) | fe(NO3) |
| DSSAT | emissions response function to soil moisture | unitless | fe(W) | fe(W) |
| DSSAT | water-filled pore space | unitless | WFPS | WFPS |
| EPIC | denitrification N2O emission rate | kg N ha−1 d−1 | DN2O | N2Odenit |
| EPIC | denitrification N2 emission rate | kg N ha−1 d−1 | DN2 | N2,denit |
| SPACSYS | denitrification N2O emitted fraction | unitless | P(N2O) | P(N2O) |
| SPACSYS | denitrification N2 emitted fraction | unitless | P(N2) | P(N2) |
| SPACSYS | adsorption factor | unitless | AD | fe(AD) |
| SPACSYS | total porosity air-filled fraction | unitless | AP | 1-WFPS |
| SPACSYS | nitrification N2O emission rate | ng N g soil−1 d−1 | N2O | N2Onit |
| SPACSYS | soil temperature | °C | T | T |
| STICS | nitrification N2O emission rate | kg N ha−1 d−1 | N2Onit | N2Onit |
| STICS | denitrification N2O emission rate | kg N ha−1 d−1 | N2Odenit | N2Odenit |
| Model | Parameter | Unit | Original Symbol | Review Symbol | Default |
|---|---|---|---|---|---|
| APSIM | N2O emission: total nitrification ratio | unitless | k2 | rN2O/nit | 0.002 |
| APSIM | gas diffusivity | unitless | k1 | gdiff | 25.1 |
| ARMOSA | N2O emission: total nitrification ratio | unitless | fN2O | rN2O/nit | 0.002 |
| ARMOSA | gas diffusivity | unitless | diffd | gdiff | 25.1 |
| CERES-EGC | N2O emission: total nitrification ratio | unitless | C | rN2O/nit | - |
| CERES-EGC | N2O emission: total denitrification ratio | unitless | R | rN2O/denit | - |
| CoupModel | NO emission: total nitrification ratio | unitless | gmfracNO | rNO/nit | 0.004 |
| CoupModel | N2O emission: total nitrification ratio | unitless | gmfracN2O | rN2O/nit | 0.0006 |
| DAYCENT | gas diffusivity | unitless | DFC or D/D0 | gdiff | - |
| DAYCENT | N2O emission: total nitrification ratio | unitless | K2 | rN2O/nit | 0.02 |
| DNDC | N2O emission: total nitrification ratio | unitless | 0.0024 | rN2O/nit | 0.0024 |
| DSSAT | N2O emission: total nitrification ratio | unitless | 0.001 | rN2O/nit | 0.001 |
| DSSAT | gas diffusivity | unitless | DFC | gdiff | - |
| DSSAT | gas diffusivity | unitless | dD0 | gdiff | - |
| EPIC | N2 emission: total denitrification | unitless | PARM(80) | rN2/denit | [0.1–0.9] |
| STICS | N2O emission: total nitrification ratio | unitless | RATIONITS | rN2O/nit | - |
| STICS | N2O emission: total denitrification ratio | unitless | RATIODENITS | rN2O/denit | - |
| Function | Model | Parameter | Unit | Original Symbol | Review Symbol | Default |
|---|---|---|---|---|---|---|
| f(T) | ARMOSA | response to a 10 °C T change | unitless | Q10 | tQ10 | 2 [1.5–4.0] |
| f(T) | ARMOSA | base T for the microbial activity | °C | Tbase | Topt | 20 |
| f(T) | CERES-EGC | nit. response to a 10 °C T change | unitless | Q10nit | tQ10 | 2.1 |
| f(T) | CERES-EGC | denit. threshold T | °C | TTrdenit | Tdenit | 11 |
| f(T) | CERES-EGC | denit. response to a 10 °C T change | unitless | Q10denit,1 | tQ10,1 | 89 |
| f(T) | CERES-EGC | denit. response to a 10 °C T change | unitless | Q10denit,2 | tQ10,2 | 2.1 |
| f(T) | CoupModel | nit./denit. cardinal Tmin | °C | tmin | Tmin | −8 |
| f(T) | CoupModel | nit./denit. cardinal Tmax | °C | tmax | Tmax | 20 |
| f(T) | CoupModel | base T for microbial activity | °C | tQ10base | Topt | 20 |
| f(T) | CoupModel | Q10 threshold | °C | tQ10thres | TQ10thres | 5 |
| f(T) | CoupModel | response to a 10 °C T change | unitless | tQ10 | tQ10 | 2 |
| f(T) | CoupModel | emissions Tmax | °C | gTmaxNxO | gTmaxNxO | 33.5 |
| f(T) | CoupModel | emissions Topt | °C | gToptNxO | gToptNxO | 23.5 |
| f(T) | CoupModel | response function shape coefficient | unitless | gTshapeNxO | gTshapeNxO | 1.5 |
| f(T) | CROPSYST | nitrification cardinal Topt | °C | TEMBAS | Topt,nit | 20 |
| f(T) | CROPSYST | response to a 10 °C T change | unitless | TEMQ10 | tQ10 | 3 [1.5–4.0] |
| f(T) | DNDC | denitrification cardinal Tmax | °C | 60 | Tmax,denit | 60 |
| f(T) | STICS | nitrification cardinal Tmin | °C | TNITMING | Tmin,nit | 5 |
| f(T) | STICS | nitrification cardinal Topt | °C | TNITOPTG | Topt,nit | 20 |
| f(T) | STICS | nitrification cardinal Tmax | °C | TNITMAXG | Tmax,nit | 45 |
| f(T) | STICS | denitrification cardinal T1 | °C | TDENREF1G | T1,denit | 11 |
| f(T) | STICS | denitrification cardinal T2 | °C | TDENREF2G | T2,denit | 20 |
| f(W) | APSIM | SWC at which denitrification ceases | m3 m−3 | SWClim | SWClim | - |
| f(W) | APSIM | empirical coefficient | unitless | X | x | 1 [0.9–5.0] |
| f(W) | ARMOSA | empirical coefficient | unitless | denitWC | x | 1 [0.9–5.0] |
| f(W) | ARMOSA | microbial activity below SWCmin | unitless | fmin | fmin | 0 |
| f(W) | ARMOSA | microbial activity above SWCmax | unitless | fmax | fmax | 0.5 |
| f(W) | ARMOSA | response function shape coefficient | unitless | A | a | 1 |
| f(W) | ARMOSA | response function shape coefficient | unitless | B | b | 1 |
| f(W) | ARMOSA | saturation threshold | unitless | thrsat | thrsat | 0.6 |
| f(W) | ARMOSA | lower threshold | unitless | thrdenit | thrdenit | 0.05 |
| f(W) | CERES-EGC | minimum WFPS for nitrification | % | MINWFPS | WFPSmin,nit | 0.1 |
| f(W) | CERES-EGC | optimum WFPS for nitrification | % | OPTWFPS | WFPSopt,nit | 0.6 |
| f(W) | CERES-EGC | maximum WFPS for nitrification | % | MAXWFPS | WFPSmax,nit | 0.8 |
| f(W) | CERES-EGC | threshold WFPS for denitrification | % | TrWFPS | WFPSdenit | 0.62 |
| f(W) | CERES-EGC | denit. response function exponent | unitless | POWdenit | x | 1.74 |
| f(W) | CoupModel | SWC effect on denit. coefficient | unitless | pθDp | pθDp | 10 |
| f(W) | CoupModel | SWC range from saturation | % | pθDRange | pθDRange | 10 |
| f(W) | CoupModel | saturation activity | unitless | pθsatact | pθsatact | 0.6 [0–1] |
| f(W) | CoupModel | water content interval lower limit | % | pθLow | pθLow | 13 [8–15] |
| f(W) | CoupModel | function shape coefficient | unitless | pθp | pθp | 1 |
| f(W) | CoupModel | water content interval upper limit | % | pθUpp | pθUpp | 8 [1–10] |
| f(W) | CoupModel | relative saturation level | unitless | gθsatcrit | gθsatcrit | Table A8 |
| f(W) | CoupModel | function shape coefficient | unitless | gθsatform | gθsatform | Table A8 |
| f(W) | CROPSYST | saturation activity | unitless | MOSSA | pθsatact | 0.6 [0–1] |
| f(W) | CROPSYST | function shape coefficient | unitless | MOSM | pθp | 1 |
| f(W) | CROPSYST | water content interval lower limit | % | MOS(1) | pθLow | 13 [8–15] |
| f(W) | CROPSYST | water content interval upper limit | % | MOS(2) | pθUpp | 8 [1–10] |
| f(W) | SPACSYS | soil bulk density | g cm−3 | ρd | BD | - |
| f(W) | SPACSYS | soil particle density | g cm−3 | ρs | PD | - |
| f(W) | STICS | optimal SWC for nitrification | unitless | HOPTNG | SWCopt,nit | 1.0 |
| f(W) | STICS | minimum SWC for nitrification | unitless | HIMINNG | SWCmin,nit | 0.67 |
| f(W) | STICS | reference T for mineralization | °C | TREFG | Tminer | 15 |
| f(W) | STICS | soil bulk density | g cm−3 | DA | BD | - |
| f(pH) | APSIM | nitrification pH minimum threshold | unitless | - | pHmin | 4.5 |
| f(pH) | APSIM | nit. pH optimum minimum value | unitless | - | pHoptmin | 6 |
| f(pH) | APSIM | nit. pH optimum maximum value | unitless | - | pHoptmax | 8 |
| f(pH) | APSIM | nitrification pH maximum threshold | unitless | - | pHmax | 9 |
| f(pH) | ARMOSA | nitrification pH minimum threshold | unitless | PHMIN | pHmin | 3 |
| f(pH) | ARMOSA | nitrification pH maximum threshold | unitless | PHMAX | pHmax | 5.5 |
| f(pH) | CoupModel | pH half rate | unitless | dpHrate | dpHrate | 4.25 |
| f(pH) | CoupModel | shape coefficient | unitless | dpHshape | dpHshape | 0.5 |
| f(pH) | CoupModel | nitrification pH minimum threshold | unitless | gpHcoeff | pHmin | 4.7 |
| f(pH) | CROPSYST | nitrification pH minimum threshold | unitless | PHMIN | pHmin | - |
| f(pH) | CROPSYST | nitrification pH maximum threshold | unitless | PHMAX | pHmax | - |
| f(pH) | DAYCENT | parameter of arctan function | unitless | A | a | - |
| f(pH) | DSSAT | nitrification pH minimum threshold | unitless | - | pHmin | - |
| f(pH) | DSSAT | nitrification pH optimum threshold | unitless | - | pHopt | - |
| f(pH) | EPIC | nit. pH optimum minimum value | unitless | - | pHoptmin | 7 |
| f(pH) | EPIC | nit. pH optimum maximum value | unitless | - | pHoptmax | 7.4 |
| f(pH) | STICS | nitrification pH minimum threshold | unitless | PHMINNITG | pHmin | 3 |
| f(pH) | STICS | nitrification pH maximum threshold | unitless | PHMAXNITG | pHmax | 5.5 |
| f(S) | CERES-EGC | nit. half-saturation constant | mg N kg−1 | Kmnit | Km,nit | 10 |
| f(S) | CERES-EGC | denit. half-saturation constant | mg N kg−1 | Kmdenit | Km,denit | 22 |
| f(S) | CoupModel | half-saturation constant | mg N L−1 | dNhalfsat | Km | 10 [5–15] |
| f(S) | CoupModel | layer thickness | m | ∆z | zlayer | - |
| f(S) | CoupModel | NO3:NH4 ratio | unitless | rnitr,amm | rNO3/NH4 | 8 [1–15] |
| f(S) | CoupModel | nit. coefficient | d−1 | nrate | knit | 0.2 |
| f(S) | CoupModel | nit. half-saturation constant | mg N L−1 | nhrateNH | Km | 6.18 |
| f(S) | DSSAT | minimum nitrate amount | kg N ha−1 d−1 | min_nitrate | NO3,min | 0.1 |
| f(S) | SPACSYS | half-saturation constant | g C m−3 or g N m−3 | km | Km | [9.45–18.53] |
| Model | Module | Parameter | NO3− Value | NO2− Value | NO Value | N2O Value |
|---|---|---|---|---|---|---|
| CoupModel | emissions | gθsatcrit | - | - | 0.45 | 0.55 |
| CoupModel | emissions | gθsatform | - | - | 0.024 | 0.24 |
| CoupModel | microbial biomass | γd,NxOy | 16 | 16 | 8.2 | 8.2 |
| CoupModel | microbial biomass | deffNxOy | 0.401 | 0.428 | 0.428 | 0.151 |
| CoupModel | microbial biomass | drcNxOy | 2.2 | 0.84 | 0.84 | 1.9 |
| SPACSYS | microbial biomass | γgd | 13.65 | 7.83 | 8.28 | 8.81 |
| SPACSYS | denitrification | YNxOy | 0.65 | 0.17 | 0.75 | 0.24 |
| SPACSYS | denitrification | MNxOy | 2.16 | 8.38 | 1.90 | 1.90 |
| Model | Ref. | Ev. | Coun. | K. C. | Crop | Meas. | Fit. | n° | Tr. | r | R2 | RMSE | RMSE95 | EF | RRMSE | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APSIM | [16] | CAL. | CHN | Cfb | corn | C | d | 2 | Nmin | - | 0.31 | - | - | - | - | - |
| [16] | CAL. | CHN | Cfb | corn | C | d | 2 | Nmin | - | 0.52 | - | - | - | - | - | |
| [16] | CAL. | CHN | Cfb | corn | C | d | 2 | Nmin | - | 0.56 | - | - | - | - | - | |
| [16] | CAL. | CHN | Cfb | corn | C | c | 2 | Nmin | - | 0.3 | - | - | - | - | - | |
| [16] | CAL. | CHN | Cfb | corn | C | c | 2 | Nmin | - | 0.67 | - | - | - | - | - | |
| [16] | CAL. | CHN | Cfb | corn | C | c | 2 | Nmin | - | 0.5 | - | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | c | - | Overall | - | 0.74 | 0.71 | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | c | - | Overall | - | 0.76 | 0.63 | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | d | 1 | N0 | - | 0.05 | 0.01 | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | d | 2 | Nmin | - | - | - | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | d | 3 | Nmin | - | 0.38 | 0.03 | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | d | 4 | Norg + Nmin | - | 0.4 | 0.03 | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | d | 1 | N0 | - | 0.08 | 0.01 | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | d | 2 | Nmin | - | - | - | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | d | 3 | Nmin | - | 0.45 | 0.03 | - | - | - | - | |
| [16] | VAL. | CHN | Cfb | winter wheat–corn | C | d | 4 | Norg + Nmin | - | 0.47 | 0.02 | - | - | - | - | |
| CERES-EGC | [90] | VAL. | IT | Csa | faba beans | C | d | 1 | Norg | 0.74 | 0.002 | 2.09 × 10−3 | - | |||
| [89] | VAL. | SW | Cfb | sugar beet–winter wheat | N | d | 1 | N0 | 0.37 | 0.006 | 6.18 × 10−3 | - | - | - | ||
| CoupModel | [7] | CAL. | SE | Cfb | red clover–winter wheat | N | c | 1–16 | Nmin + SWC | - | 0.96 | 0.82 | - | - | 275 | - |
| [7] | CAL. | SE | Cfb | red clover–winter wheat | N | c | 16 | Overall | - | 0.47 | 0.71 | - | - | 278 | - | |
| [91] | VAL. | DE | Cfb | rapeseed | N | d | 1 | N0 | - | 0.26 | 0.04 | - | - | - | - | |
| [91] | VAL. | DE | Cfb | rapeseed | N | d | 2 | Nmin | - | 0.18 | 0.04 | - | - | - | - | |
| [91] | VAL. | DE | Cfb | rapeseed | N | d | 3 | Nmin | - | 0.5 | 0.05 | - | - | - | - | |
| DAYCENT | [11] | VAL. | USA | Bsk | grassland | N | c | 1 | SL | 0.35 | - | - | - | - | 0.045 | |
| [11] | VAL. | USA | Bsk | grassland | N | c | 3 | SCL | - | 0.18 | - | - | - | - | 0.19 | |
| [11] | VAL. | USA | Bsk | grassland | N | c | 2 | Nmin + SL | - | 0.46 | - | - | - | - | 0.16 | |
| [11] | VAL. | USA | Bsk | grassland | N | c | 5 | CL | - | 0.64 | - | - | - | - | 0.01 | |
| [11] | VAL. | USA | Bsk | grassland | N | c | - | Overall | - | 0.26 | - | - | - | - | 0.0001 | |
| [93] | VAL. | USA | Bsk | corn | N | c | 1 | Overall | - | 0.29 | - | - | - | - | - | |
| [92] | VAL. | CH | Cfb | rotation 1 | N | c | 1 | Overall | - | 0.89 | 1.04 | - | - | 25 | - | |
| [11] | VAL. | USA | Bsk | grassland | N | c | 4 | Nmin + SCL | - | 0.02 | - | - | - | - | 0.69 | |
| [11] | VAL. | USA | Bsk | grassland | N | d | 1 | SL | 0.07 | - | - | - | - | 0.0001 | ||
| [11] | VAL. | USA | Bsk | grassland | N | d | 3 | SCL | - | 0.08 | - | - | - | - | 0.0006 | |
| [11] | VAL. | USA | Bsk | grassland | N | d | 2 | Nmin + SL | - | 0.19 | - | - | - | - | 0.0001 | |
| [11] | VAL. | USA | Bsk | grassland | N | d | 5 | CL | - | 0.02 | - | - | - | - | 0.1 | |
| [11] | VAL. | USA | Bsk | grassland | N | d | - | Overall | - | 0.09 | - | - | - | - | 0.0001 | |
| [11] | VAL. | USA | Bsk | grassland | N | d | 4 | Nmin + SCL | - | 0.02 | - | - | - | - | 0.14 | |
| DNDC v.9.5 | [95] | VAL. | CHN | Cfa | rotation 3 | C | c | - | Overall | - | 0.95 | - | - | - | - | - |
| [94] | CAL. | CHN | Cfa | rotation 2 | N | c | 4 | Overall | - | 0.75 | 0.51 | - | - | - | - | |
| [95] | VAL. | CHN | Cfa | rotation 3 | C | d | 1 | Norg + Nmin | - | 0.4 | - | - | - | - | - | |
| [95] | VAL. | CHN | Cfa | rotation 3 | C | d | 2 | Norg + Nmin | - | 0.28 | - | - | - | - | - | |
| [94] | CAL. | CHN | Cfa | rotation 2 | N | d | 1 + 2 | Cropmng (rice) | - | - | 0.01–0.03 | - | - | - | - | |
| [94] | CAL. | CHN | Cfa | rotation 2 | N | d | 3 + 4 | Cropmng (vegetable) | - | - | 0.11 | - | - | - | - | |
| [95] | VAL. | CHN | Cfa | rotation 3 | C | d | 3 | Norg + Nmin + nitr. inhibitor | - | 0.3 | - | - | - | - | - | |
| EPIC | [96] | VAL. | USA | Dfa | rotation 5 | N | c | 1–7 | (4) Nmin + (3) Cropmng | - | 0.54 | 1.32 | - | - | - | - |
| [82] | CAL. | USA | Dfa | rotation 4 | N | c | - | Overall (2007) | - | 0.88 | 0.67 | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | c | - | Overall (2008) | - | 0.78 | 0.39 | - | - | - | - | |
| [96] | CAL. | USA | Dfa | rotation 5 | N | c | 1–7 | (4) Nmin + (3) Cropmng | - | 0.41 | 1.25 | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 1 | N0 (2007) | - | 0.31 | - | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 2 | Nmin (2007) | - | 0.63 | - | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 3 | Nmin (2007) | - | 0.82 | - | - | - | - | - | |
| EPIC | [82] | CAL. | USA | Dfa | rotation 4 | N | d | 4 | Nmin (2007) | - | 0.78 | - | - | - | - | - |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 5 | Nmin (2007) | - | 0.58 | - | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 6 | Nmin (2007) | - | 0.7 | - | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | - | Overall (2007) | - | 0.92 | 0 | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 1 | N0 (2008) | - | 0.1 | - | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 2 | Nmin (2008) | - | 0.32 | - | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 3 | Nmin (2008) | - | 0.55 | - | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 4 | Nmin (2008) | - | 0.77 | - | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 5 | Nmin (2008) | - | 0.64 | - | - | - | - | - | |
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | 6 | Nmin (2008) | - | 0.75 | - | - | - | - | ||
| [82] | CAL. | USA | Dfa | rotation 4 | N | d | - | Overall (2008) | - | 0.78 | 0.04 | - | - | - | - | |
| [96] | CAL. | USA | Dfa | rotation 5 | N | d | 1–7 | (4) Nmin + (3) Cropmng | - | 0.45 | 0.01 | - | - | - | - | |
| [96] | VAL. | USA | Dfa | rotation 5 | N | d | 1–7 | (4) Nmin + (3) Cropmng | - | 0.14 | 0.02 | - | - | - | - | |
| SPACSYS | [98] | VAL. | U.K. | Cfb | permanent pasture | C | d | 1 | Nmin | 0.49 | 0.74 | 0.07 | - | −0.2 | - | - |
| [71] | VAL. | GB–SCT | Cfb | grassland | N | c | 1 | N0 | 0.06 | - | 0.494 | 0.87 | −22.25 | - | - | |
| [71] | VAL. | GB–SCT | Cfb | grassland | N | c | 3 | Norg | 0.5 | - | 0.177 | 0.747 | 0.2 | - | - | |
| [71] | VAL. | GB–SCT | Cfb | grassland | N | c | 2 | Nmin | 0.34 | - | 0.52 | 0.736 | 0.04 | - | - | |
| [97] | VAL. | U.K. | Cfb | grassland | N | c | 1 | Nmin | 0.98 | - | 0.1305 | 0.17415 | - | - | - | |
| [97] | CAL. | U.K. | Cfb | grassland | N | c | 1 | Nmin | 0.88 | - | 0.3841 | 0.24733 | - | - | - | |
| STICS | [99] | VAL. | SP, SP–C, FR | Cfa (SP), Csa (SP-C), Cfb (FR) | Durum wheat –faba bean | C | c | 12 | Nmin + Cropmng | - | 0.4 | - | - | 0.24 | 45.6 |
References
- Mosier, A.; Kroeze, C. Potential impact on the global atmospheric N2O budget of the increased nitrogen input required to meet future global food demands. Chemosphere Glob. Chang. Sci. 2000, 2, 465–473. [Google Scholar] [CrossRef]
- Monson, R.K.; Holland, E.A. Biospheric Trace Gas Fluxes and Their Control Over Tropospheric Chemistry. Annu. Rev. Ecol. Syst. 2001, 32, 547–576. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Northrup, D.L.; Basso, B.; Wang, M.Q.; Morgan, C.L.S.; Benfey, P.N. Novel technologies for emission reduction complement conservation agriculture to achieve negative emissions from row-crop production. Proc. Natl. Acad. Sci. USA 2021, 118, e2022666118. [Google Scholar] [CrossRef] [PubMed]
- Borken, W.; Matzner, E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob. Chang. Biol. 2009, 15, 808–824. [Google Scholar] [CrossRef]
- Stein, L.Y.; Nicol, G.W. Nitrification. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Jansson, P.-E.; Petersen, S.O. Modeling nitrous oxide emissions from agricultural soil incubation experiments using CoupModel. Biogeosciences 2022, 19, 4811–4832. [Google Scholar] [CrossRef]
- Pu, Y.; Zhu, B.; Dong, Z.; Liu, Y.; Wang, C.; Ye, C. Soil N2O and NOx emissions are directly linked with N-cycling enzymatic activities. Appl. Soil Ecol. 2019, 139, 15–24. [Google Scholar] [CrossRef]
- Smith, K.A. Changing views of nitrous oxide emissions from agricultural soil: Key controlling processes and assessment at different spatial scales. Eur. J. Soil Sci. 2017, 68, 137–155. [Google Scholar] [CrossRef]
- Ottaiano, L.; Di Mola, I.; Di Tommasi, P.; Mori, M.; Magliulo, V.; Vitale, L. Effects of irrigation on N2O emissions in a maize crop grown on different soil types in two contrasting seasons. Agriculture 2020, 10, 623. [Google Scholar] [CrossRef]
- Parton, W.J.; Holland, E.A.; Del Grosso, S.J.; Hartman, M.D.; Martin, R.E.; Mosier, A.R.; Ojima, D.S.; Schimel, D.S. Generalized model for NOx and N2O emissions from soils. J. Geophys. Res. 2001, 106, 17403–17419. [Google Scholar] [CrossRef]
- Weier, K.L.; Doran, J.W.; Power, J.F.; Walters, D.T. Denitrification and the Dinitrogen/Nitrous Oxide Ratio as Affected by Soil Water, Available Carbon, and Nitrate. Soil Sci. Soc. Am. J. 1993, 57, 66–72. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Hénault, C.H.; Grossel, A.; Mary, B.; Roussel, M.; Eonard, J.L. Nitrous Oxide Emission by Agricultural Soils: A Review of Spatial and Temporal Variability for Mitigation. Pedosphere 2012, 22, 426–433. [Google Scholar] [CrossRef]
- Maag, M.; Vinther, F.P. Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil. Appl. Soil Ecol. 1996, 4, 5–14. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Luo, Z.; Wang, E.; Wang, G.; Zhou, H.; Li, H.; Xu, S. Reducing N2O emissions while maintaining yield in a wheat–maize rotation system modelled by APSIM. Agric. Syst. 2021, 194, 103277. [Google Scholar] [CrossRef]
- Del Grosso, S.J.; Ojima, D.S.; Parton, W.J.; Stehfest, E.; Heistemann, M.; DeAngelo, B.; Rose, S. Global scale DAYCENT model analysis of greenhouse gas emissions and mitigation strategies for cropped soils. Glob. Planet. Chang. 2009, 67, 44–50. [Google Scholar] [CrossRef]
- Schwenke, G.D.; Haigh, B.M. Can split or delayed application of N fertiliser to grain sorghum reduce soil N2O emissions from sub-tropical Vertosols and maintain grain yields? Soil Res. 2019, 57, 859–874. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Toosi, E.R.; Guber, A.K.; Ostrom, N.E.; Yu, J.; Azeem, K.; Rivers, M.L.; Robertson, G.P. Hotspots of soil N2O emission enhanced through water absorption by plant residue. Nat. Geosci. 2017, 10, 496–500. [Google Scholar] [CrossRef]
- Miller, L.T.; Griffis, T.J.; Erickson, M.D.; Turner, P.A.; Deventer, M.J.; Chen, Z.; Yu, Z.; Venterea, R.T.; Baker, J.M.; Frie, A.L. Response of nitrous oxide emissions to individual rain events and future changes in precipitation. J. Environ. Qual. 2022, 51, 312–324. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycles 2002, 16, 6-1–6-13. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobeña, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef]
- Li, R.; Cameira, M.R.; Fangueiro, D. Modelling nitrous oxide emissions from an oats cover crop with the RZWQM2: In Advances in Agricultural Systems Modeling 8. Bridging Among Disciplines by Synthesizing Soil and Plant Processes; Li, R., Cameira, M.R., Fangueiro, D., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Pattey, E.; Edwards, G.C.; Desjardins, R.L.; Pennock, D.J.; Smith, W.; Grant, B.; MacPherson, J.I. Tools for quantifying N2O emissions from agroecosystems. Agric. For. Meteorol. 2007, 142, 103–119. [Google Scholar] [CrossRef]
- Beheydta, D.; Boeckxa, P.; Sleutelb, S.; Lic, C.; Van Cleemput, O. Validation of DNDC for 22 long-term N2O field emission measurements. Atmos. Environ. 2007, 41, 6196–6211. [Google Scholar] [CrossRef]
- Flessa, H.; Ruser, R.; Schilling, R.; Loftfield, N.; Munch, J.C.; Kaiser, E.A.; Beese, F. N2O and CH4 fluxes in potato fields: Automated 24 measurement, management effects and temporal variation. Geoderma 2002, 105, 307–325. [Google Scholar] [CrossRef]
- Smith, K.A.; Dobbie, K.E. The impact of sampling frequency and sampling times on chamber-based measurements of N2O emissions from fertilized soils. Glob. Chang. Biol. 2001, 7, 933–945. [Google Scholar] [CrossRef]
- Rochette, P.; Desjardins, R.L.; Pattey, E.; Lessard, R. Instantaneous Measurement of Radiation and Water Use Efficiencies of a Maize Crop. Agron. J. 1996, 88, 627–635. [Google Scholar] [CrossRef]
- Eugster, W.; Merbold, L. Eddy covariance for quantifying trace gas fluxes from soils. Soil 2015, 1, 187–205. [Google Scholar] [CrossRef]
- Grant, R.F.; Pattey, E. Modelling variability in N2O emissions from fertilized agricultural fields. Soil Biol. Biochem. 2003, 35, 225–243. [Google Scholar] [CrossRef]
- Cowan, N.J.; Norman, P.; Famulari, D.; Levy, P.E.; Reay, D.S.; Skiba, U.M. Spatial variability and hotspots of soil N2O fluxes from intensively grazed grassland. Biogeosciences 2015, 12, 1585–1596. [Google Scholar] [CrossRef]
- Denmead, O.T. Micrometeorological methods for measuring gaseous losses of nitrogen in the field. In Gaseous Loss of Nitrogen from Plant-Soil Systems. Developments in Plant and Soil Sciences; Freney, J.R., Simpson, J.R., Eds.; Springer: Dordrecht, The Netherlands, 1983; Volume 9. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Hincks, B.B.; Meyers, T.P. Measuring Biosphere-Atmosphere Exchanges of Biologically Related Gases with Micrometeorological Methods. Ecology 1988, 69, 1331–1340. [Google Scholar] [CrossRef]
- Foken, T.; Aubinet, M.; Leuning, R. The Eddy Covariance Method. In A Practical Guide to Measurement and Data Analysis; Aubinet, M., Vesala, T., Papale, D., Eds.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK; New York, NY, USA, 2012; ISBN 978-94-007-2350-4. [Google Scholar]
- Shi, R.; Su, P.; Zhou, Z.; Yang, J.; Ding, X. Comparison of eddy covariance and automatic chamber-based methods for measuring carbon flux. Agron. J. 2022, 114, 2081–2094. [Google Scholar] [CrossRef]
- Liang, L.L.; Campbell, D.I.; Wall, A.M.; Schipper, L.A. Nitrous oxide fluxes determined by continuous eddy covariance measurements from intensively grazed pastures: Temporal patterns and environmental controls. Agric. Ecosyst. Environ. 2018, 268, 171–180. [Google Scholar] [CrossRef]
- Shurpali, N.J.; Rannik, Ü.; Jokinen, S.; Lind, S.; Biasi, C.; Mammarella, I.; Peltola, O.; Pihlatie, M.; Hyvönen, N.; Räty, M.; et al. Neglecting diurnal variations leads to uncertainties in terrestrial nitrous oxide emissions. Sci. Rep. 2016, 6, 25739. [Google Scholar] [CrossRef]
- Giltrap, D.L.; Kirschbaum, M.U.; Liáng, L.L. The potential effectiveness of four different options to reduce environmental impacts of grazed pastures. A model-based assessment. Agric. Syst. 2021, 186, 102960. [Google Scholar] [CrossRef]
- Uddin, S.; Islam, M.R.; Jahangir, M.M.R.; Rahman, M.M.; Hassan, S.; Hassan, M.M.; Abo-Shosha, A.A.; Ahmed, A.F.; Rahman, M.M. Nitrogen release in soils amended with different organic and inorganic fertilizers under contrasting moisture regimes: A laboratory incubation study. Agronomy 2021, 11, 2163. [Google Scholar] [CrossRef]
- Schädel, C.; Beem-Miller, J.; Aziz Rad, M.; Crow, S.E.; Hicks Pries, C.E.; Ernakovich, J.; Hoyt, A.M.; Plante, A.; Stoner, S.; Treat, C.C.; et al. Decomposability of soil organic matter over time: The Soil Incubation Database (SIDb, version 1.0) and guidance for incubation procedures. Earth Syst. Sci. Data 2020, 12, 1511–1524. [Google Scholar] [CrossRef]
- Lapitan, R.; Wanninkhof, R.; Mosier, A. Methods for stable gas flux determination in aquatic and terrestrial systems. Dev. Atmos. Sci. 1999, 24, 29–66. [Google Scholar] [CrossRef]
- Avadí, A.; Galland, V.; Versini, A.; Bockstaller, C. Suitability of operational N direct field emissions models to represent contrasting agricultural situations in agricultural LCA: Review and prospectus. Sci. Total Environ. 2022, 802, 149960. [Google Scholar] [CrossRef]
- Dorich, C.D.; Conant, R.T.; Albanito, F.; Butterbach-Bahl, K.; Grace, P.; Scheer, C.; Snow, V.O.; Vogeler, I.; van der Weerden, T.J. Improving N2O emission estimates with the global N2O database. Curr. Opin. Environ. Sustain. 2020, 47, 13–20. [Google Scholar] [CrossRef]
- Nemecek, T.; Gaillard, G. Challenges in assessing the environmental impacts of crop production and horticulture. In Environmental Assessment and Management in the Food Industry—Life Cycle Assessment and Related Approaches; Sonesson, U., Berlin, J., Ziegler, F., Eds.; Woodhead Publishing Limited: Oxford, UK, 2010; Chapter 6; pp. 98–116. [Google Scholar]
- Nevison, C. Review of the IPCC methodology for estimating nitrous oxide emissions associated with agricultural leaching and runoff. Chemosphere Glob. Chang. Sci. 2000, 2, 493–500. [Google Scholar] [CrossRef]
- Shang, Z.; Abdalla, M.; Kuhnert, M.; Albanito, F.; Zhou, F.; Xia, L.; Smith, P. Measurement of N2O emissions over the whole year is necessary for estimating reliable emission factors. Environ. Pollut. 2020, 259, 113864. [Google Scholar] [CrossRef]
- Yan, H.P. A Dynamic, Architectural Plant Model Simulating Resource-dependent Growth. Ann. Bot. 2004, 93, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.F. Direct emission of nitrous oxide from agricultural soils. Nutr. Cycl. Agroecosystems 1996, 46, 53–70. [Google Scholar] [CrossRef]
- Freibauer, A.; Kaltschmitt, M. Controls and models for estimating direct nitrous oxide emissions from temperate and sub-boreal agricultural mineral soils in Europe. Biogeochemistry 2003, 63, 93–115. [Google Scholar] [CrossRef]
- Tonitto, C.; Woodbury, P.B.; McLellan, E.L. Defining a best practice methodology for modeling the environmental performance of agriculture. Environ. Sci. Policy 2018, 87, 64–73. [Google Scholar] [CrossRef]
- Mummey, D.L.; Smith, J.L.; Bluhm, G. Assessment of alternative soil management practices on N2O emissions from US agriculture. Agric. Ecosyst. Environ. 1998, 70, 79–87. [Google Scholar] [CrossRef]
- Li, C.S. Modeling Trace Gas Emissions from Agricultural Ecosystems. Nutr. Cycl. Agroecosystems 2000, 58, 259–276. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Grace, P.; Mosier, A.R. N2O emissions from agricultural lands: A synthesis of simulation approaches. Plant Soil 2008, 309, 169–189. [Google Scholar] [CrossRef]
- Brilli, L.; Bechini, L.; Bindi, M.; Carozzi, M.; Cavalli, D.; Conant, R.; Dorich, C.D.; Doro, L.; Ehrhardt, F.; Farina, R.; et al. Review and analysis of strengths and weaknesses of agro-ecosystem models for simulating C and N fluxes. Sci. Total Environ. 2017, 598, 445–470. [Google Scholar] [CrossRef]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Perego, A.; Giussani, A.; Sanna, M.; Fumagalli, M.; Carozzi, M.; Alfieri, L.; Brenna, S.; Acutis, M. The ARMOSA simulation crop model: Overall features, calibration and validation results. Ital. J. Agrometeorol. 2013, 3, 23–38. [Google Scholar]
- Holzworth, D.P.; Huth, N.I.; deVoil, P.G.; Zurcher, E.J.; Herrmann, N.I.; McLean, G.; Chenu, K.; van Oosterom, E.J.; Snow, V.; Murphy, C.; et al. APSIM—Evolution towards a new generation of agricultural systems simulation. Environ. Model. Softw. 2014, 62, 327–350. [Google Scholar] [CrossRef]
- Gabrielle, B.; Da-Silveira, J.; Houot, S.; Michelin, J. Field-scale modelling of carbon and nitrogen dynamics in soils amended with urban waste composts. Agric. Ecosyst. Environ. 2005, 110, 289–299. [Google Scholar] [CrossRef]
- Stöckle, C.O.; Donatelli, M.; Nelson, R. CropSyst, a cropping systems simulation model. Eur. J. Agron. 2003, 18, 289–307. [Google Scholar] [CrossRef]
- Brisson, N.; Launay, M.; Mary, B.; Beaudoin, N. Conceptual Basis, Formalisations and Parameterization of the STICS Crop Model; Edition Quae: Versailles, France, 2008. [Google Scholar]
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Williams, J.R. EPIC—Erosion/Productivity Impact Calculator Model Documentation; U.S. Department of Agriculture Technical Bulletin No. 1768; USA Government Printing Office: Washington, DC, USA, 1990.
- Li, C.; Frolking, S.; Frolking, T.A. A model of nitrous oxide evolution from soil driven by rainfall events: 1. Model structure and sensitivity. J. Geophys. Res. 1992, 97, 9759–9776. [Google Scholar] [CrossRef]
- Parton, W.J.; Ojima, D.S.; Cole, C.V.; Schimel, D.S. A General Model for Soil Organic Matter Dynamics: Sensitivity to Litter Chemistry, Texture and Management. In Quantitative Modeling of Soil Forming Processes; Bryant, R.B., Arnold, R.W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994. [Google Scholar] [CrossRef]
- Jansson, P.-E. CoupModel: Model use, calibration and validation. Trans. ASABE 2012, 55, 1335–1344. [Google Scholar]
- Wu, L.; McGechan, M.B.; McRoberts, N.; Baddeley, J.A.; Watson, C.A. SPACSYS: Integration of a 3D root architecture component to carbon, nitrogen and water cycling—Model description. Ecol. Model. 2007, 200, 343–359. [Google Scholar] [CrossRef]
- Thorburn, P.J.; Biggs, J.S.; Collins, K.; Probert, M.E. Using the APSIM model to estimate nitrous oxide emissions from diverse Australian sugarcane production systems. Agric. Ecosyst. Environ. 2010, 136, 343–350. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Karlberg, L. Coupled Heat and Mass Transfer Model for Soilplant-Atmosphere Systems; Royal Institute of Technology: Stockholm, Sweden, 2010; Available online: www2.lwr.kth.se/CoupModel/coupmanual.pdf (accessed on 16 January 2024).
- Stöckle, C.; Higgins, S.; Kemanian, A.; Nelson, R.; Huggins, D.; Marcos, J.; Collins, H. Carbon storage and nitrous oxide emissions of cropping systems in eastern Washington: A simulation study. J. Soil Water Conserv. 2012, 67, 365–377. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Shelia, V.; Wilkens, P.W.; Singh, U.; White, J.W.; Asseng, S.; Lizaso, J.I.; Moreno, L.P.; et al. The DSSAT crop modeling ecosystem. In Advances in Crop Modelling for a Sustainable Agriculture; Boote, K., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 173–216. ISBN 9781786762405. [Google Scholar]
- Wu, L.; Rees, R.M.; Tarsitano, D.; Zhang, X.; Jones, S.K.; Whitmore, A.P. Simulation of nitrous oxide emissions at field scale using the SPACSYS model. Sci. Total Environ. 2015, 530–531, 76–86. [Google Scholar] [CrossRef]
- Meier, E.A.; Thorburn, P.J.; Probert, M.E. Occurrence and simulation of nitrification in two contrasting sugarcane soils from the Australian wet tropics. Aust. J. Soil Res. 2006, 44, 1–9. [Google Scholar] [CrossRef]
- Stöckle, C.O.; Martin, S.A.; Campbell, G.S. CropSyst, a cropping systems simulation model: Water/nitrogen budgets and crop yield. Agric. Syst. 1994, 46, 335–359. [Google Scholar] [CrossRef]
- Wu, L.; McGechan, M.B. A Review of Carbon and Nitrogen Processes in Four Soil Nitrogen Dynamics Models. J. Agric. Eng. Res. 1998, 69, 279–305. [Google Scholar] [CrossRef]
- Eckersten, H.; Jansson, P.-E.; Johnsson, H. SOILN Model User’s Manual: Version 9.2; Avdelningsmeddelande 98:6 Communications: Uppsala, Sweden, 1998; ISRN SLU-HY-AVDM--98/6--SE 0282-6569. [Google Scholar]
- Lehuger, S. Modélisation des Bilans de Gaz à Effet de Serre des Agro-Écosystèmes en Europe. Ph.D. Thesis, AgroParisTech, Paris, France, 2009. [Google Scholar]
- Available online: https://github.com/DSSAT/dssat-csm-os (accessed on 16 November 2023).
- EPIC v.1102. Software Executable. Available online: https://epicapex.tamu.edu/software/ (accessed on 16 November 2023).
- Institute for the Study of Earth, Oceans, and Space. DNDC Scientific Basis and Processes: Version 9.5; Institute for the Study of Earth, Oceans, and Space: Durham, NH, USA, 2017. [Google Scholar]
- Parton, W.J.; Mosier, A.R.; Ojima, D.S.; Valentine, D.W.; Schimel, D.S.; Weier, K.; Kulmala, A.E. Generalized model for N2 and N2O production from nitrification and denitrification. Glob. Biogeochem. Cycles 1996, 10, 401–412. [Google Scholar] [CrossRef]
- Del Grosso, S.J.; Parton, W.J.; Mosier, A.R.; Ojima, D.S.; Kulmala, A.E.; Phongpan, S. General model for N2O and N2 gas emissions from soils due to dentrification. Glob. Biogeochem. Cycles 2000, 14, 1045–1060. [Google Scholar] [CrossRef]
- Izaurralde, R.C.; McGill, W.B.; Williams, J.R.; Jones, C.D.; Link, R.P.; Manowitz, D.H.; Schwab, D.E.; Zhang, X.; Robertson, G.P.; Millar, N. Simulating microbial denitrification with EPIC: Model description and evaluation. Ecol. Model. 2017, 359, 349–362. [Google Scholar] [CrossRef]
- Hénault, C.; Bizouard, F.; Laville, P.; Gabrielle, B.; Nicoullaud, B.; Germon, J.C.; Cellier, P. Predicting in situ soil N2O emission using NOE algorithm and soil database. Glob. Chang. Biol. 2005, 11, 115–127. [Google Scholar] [CrossRef]
- APSIM 7.10. Soil Modules Documentation: SoilN. Available online: https://www.apsim.info/documentation/model-documentation/soil-modules-documentation/soiln/ (accessed on 16 November 2023).
- Available online: https://www.nrel.colostate.edu/projects/daycent-executables/ (accessed on 16 November 2023).
- Franzluebbers, A.J. Microbial activity in response to water-filled pore space of variably eroded southern Piedmont soils. Appl. Soil Ecol. 1999, 11, 91–101. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Arnfield, A.J. Köppen Climate Classification. Available online: https://www.britannica.com/science/Koppen-climate-classification (accessed on 16 November 2023).
- Haas, E.; Carozzi, M.; Massad, R.S.; Scheer, C.; Butterbach-Bahl, K. Testing the Performance of CERES-EGC and LandscapeDNDC to Simulate Effects of Residue Management on Soil N2O Emissions. ResidueGas Deliverable Report 4.1. 2021. Available online: https://projects.au.dk/fileadmin/projects/residuegas/D_reports/ResidueGas_D4.1.pdf (accessed on 16 November 2023).
- Ferrara, R.M.; Carozzi, M.; Decuq, C.; Loubet, B.; Finco, A.; Marzuoli, R.; Gerosa, G.; Di Tommasi, P.; Magliulo, V.; Rana, G. Ammonia, nitrous oxide, carbon dioxide, and water vapor fluxes after green manuring of faba bean under Mediterranean climate. Agric. Ecosyst. Environ. 2021, 315, 107439. [Google Scholar] [CrossRef]
- Köbke, S.; He, H.; Böldt, M.; Wang, H.; Senbayram, M.; Dittert, K. Climate Overrides Effects of Fertilizer and Straw Management as Controls of Nitrous Oxide Emissions After Oilseed Rape Harvest. Front. Environ. Sci. 2022, 9, 773901. [Google Scholar] [CrossRef]
- Necpalova, M.; Lee, J.; Skinner, C.; Büchi, L.; Wittwer, R.; Gattinger, A.; van der Heijden, M.; Mäder, P.; Charles, R.; Berner, A.; et al. Potentials to mitigate greenhouse gas emissions from Swiss agriculture. Agric. Ecosyst. Environ. 2018, 265, 84–102. [Google Scholar] [CrossRef]
- Del Grosso, S.; Parton, W.; Mosier, A.; Hartman, M.; Brenner, J.; Ojima, D.; Schimel, D. Simulated Interaction of Carbon Dynamics and Nitrogen Trace Gas Fluxes Using the DAYCENT Model. In Modeling Carbon and Nitrogen Dynamics for Soil Management; Hansen, S., Shaffer, M., Ma, L., Eds.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-1-56670-529-5. [Google Scholar]
- Sun, X.; Yang, X.; Hou, J.; Wang, B.; Fang, Q. Modeling the Effects of Rice-Vegetable Cropping System Conversion and Fertilization on GHG Emissions Using the DNDC Model. Agronomy 2023, 13, 379. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, Q.; Schadt, C.W.; Mayes, M.A.; Zhang, D.; Hui, D. Precipitation and nitrogen application stimulate soil nitrous oxide emission. Nutr. Cycl. Agroecosystems 2021, 120, 363–378. [Google Scholar] [CrossRef]
- Gaillard, R.K.; Jones, C.D.; Ingraham, P.; Collier, S.; Izaurralde, R.C.; Jokela, W.; Osterholz, W.; Salas, W.; Vadas, P.; Ruark, M.D. Underestimation of N2O emissions in a comparison of the DayCent, DNDC, and EPIC models. Ecol. Appl. 2018, 28, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Abalos, D.; Cardenas, L.M.; Wu, L. Climate change and N2O emissions from South West England grasslands: A modelling approach. Atmos. Environ. 2016, 132, 249–257. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Harris, P.; Cardenas, L.M.; Dunn, R.M.; Sint, H.; Murray, P.J.; Lee, M.R.; Wu, L. Modelling field scale spatial variation in water run-off, soil moisture, N2O emissions and herbage biomass of a grazed pasture using the SPACSYS model. Geoderma 2018, 315, 49–58. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Léonard, J.; Peyrard, C.; Mary, B.; Justes, É. Precipitation gradient and crop management affect N2O emissions: Simulation of mitigation strategies in rainfed Mediterranean conditions. Agric. Ecosyst. Environ. 2017, 238, 89–103. [Google Scholar] [CrossRef]
- Ehrhardt, F.; Soussana, J.-F.; Bellocchi, G.; Grace, P.; McAuliffe, R.; Recous, S.; Sándor, R.; Smith, P.; Snow, V.; de Antoni Migliorati, M.; et al. Assessing uncertainties in crop and pasture ensemble model simulations of productivity and N2O emissions. Glob. Chang. Biol. 2018, 24, e603–e616. [Google Scholar] [CrossRef]
- Donatelli, M.; Acutis, M.; Bellocchi, G.; Fila, G. New Indices to Quantify Patterns of Residuals Produced by Model Estimates. Agron. J. 2004, 96, 631–645. [Google Scholar] [CrossRef]
- Brown, L.; Brown, S.A.; Jarvis, S.C.; Syed, B.; Goulding, K.W.T.; Phillips, V.R.; Sneath, R.W.; Pain, B.F. An inventory of nitrous oxide emissions from agriculture in the UK using the IPCC methodology: Emission estimate, uncertainty and sensitivity analysis. Atmos. Environ. 2001, 35, 1439–1449. [Google Scholar] [CrossRef]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Seitzinger, S.; Van Cleemput, O. An overview of the revised 1996 IPCC guidelines for national greenhouse gas inventory methodology for nitrous oxide from agriculture. Environ. Sci. Policy 1999, 2, 325–333. [Google Scholar] [CrossRef]
- Lokupitiya, E.; Paustian, K. Agricultural Soil Greenhouse Gas Emissions. J. Environ. Qual. 2006, 35, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Amon, B.; Çinar, G.; Anderl, M.; Dragoni, F.; Kleinberger-Pierer, M.; Hörtenhuber, S. Inventory reporting of livestock emissions: The impact of the IPCC 1996 and 2006 Guidelines. Environ. Res. Lett. 2021, 16, 075001. [Google Scholar] [CrossRef]
- Leip, A.; Busto, M.; Winiwarter, W. Developing spatially stratified N2O emission factors for Europe. Environ. Pollut. 2011, 159, 3223–3232. [Google Scholar] [CrossRef]
- Millar, N.; Robertson, G.P.; Grace, P.R.; Gehl, R.J.; Hoben, J.P. Nitrogen fertilizer management for nitrous oxide (N2O) mitigation in intensive corn (Maize) production: An emissions reduction protocol for US Midwest agriculture. Mitig. Adapt. Strateg. Glob. Chang. 2010, 15, 185–204. [Google Scholar] [CrossRef]
- Tonitto, C.; David, M.B.; Drinkwater, L.E. Modeling N2O flux from an Illinois agroecosystem using Monte Carlo sampling of field observations. Biogeochemistry 2009, 93, 31–48. [Google Scholar] [CrossRef]
- Smith, P.; Davies, C.A.; Ogle, S.; Zanchi, G.; Bellarby, J.; Bird, N.; Boddey, R.M.; McNamara, N.P.; Powlson, D.; Cowie, A.; et al. Towards an integrated global framework to assess the impacts of land use and management change on soil carbon: Current capability and future vision. Glob. Chang. Biol. 2012, 18, 2089–2101. [Google Scholar] [CrossRef]
- Basche, A.D.; Miguez, F.E.; Kaspar, T.C.; Castellano, M.J. Do cover crops increase or decrease nitrous oxide emissions? a meta-analysis. J. Soil Water Conserv. 2014, 69, 471–482. [Google Scholar] [CrossRef]
- Van Kessel, C.; Venterea, R.; Six, J.; Adviento-Borbe, M.A.; Linquist, B.; Jan van Groenigen, K. Climate, duration, and N placement determine N2O emissions in reduced tillage systems: A meta-analysis. Glob. Chang. Biol. 2013, 19, 33–44. [Google Scholar] [CrossRef]
- Villa-Vialaneix, N.; Follador, M.; Ratto, M.; Leip, A. A comparison of eight metamodeling techniques for the simulation of N2O fluxes and N leaching from corn crops. Environ. Model. Softw. 2012, 34, 51–66. [Google Scholar] [CrossRef]
- Robertson, G.P.; Paul, E.A.; Harwood, R.R. Greenhouse Gases in Intensive Agriculture: Contributions of Individual Gases to the Radiative Forcing of the Atmosphere. Science 2000, 289, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- Del Prado, A.; Crosson, P.; Olesen, J.E.; Rotz, C.A. Whole-farm models to quantify greenhouse gas emissions and their potential use for linking climate change mitigation and adaptation in temperate grassland ruminant-based farming system. Animal 2013, 7 (Suppl. 2), 373–385. [Google Scholar] [CrossRef] [PubMed]
- Louhichi, K.; Kanellopoulos, A.; Janssen, S.; Flichman, G.; Blanco, M.; Hengsdijk, H.; Heckelei, T.; Berentsen, P.; Lansink, A.O.; Van Ittersum, M. FSSIM, a bio-economic farm model for simulating the response of EU farming systems to agricultural and environmental policies. Agric. Syst. 2010, 103, 585–597. [Google Scholar] [CrossRef]
- Shang, L.; Wang, J.; Schäfer, D.; Heckelei, T.; Gall, J.; Appel, F.; Storm, H. Surrogate modelling of a detailed farm-level model using deep learning. J. Agric. Econ. 2023, 1–26. [Google Scholar] [CrossRef]
- Cann, D.J.; Hunt, J.R.; Malcolm, B. Long fallows can maintain whole-farm profit and reduce risk in semi-arid south-eastern Australia. Agric. Syst. 2020, 178, 102721. [Google Scholar] [CrossRef]
- Loague, K.; Green, R.E. Statistical and graphical methods for evaluating solute transport models: Overview and application. J. Contam. Hydrol. 1991, 7, 51–73. [Google Scholar] [CrossRef]
- Nash, J.E.; Sutcliffe, J.V. River flow forecasting through conceptual models part I—A discussion of principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Addiscott, T.M.; Whitmore, A.P. Computer simulation of changes in soil mineral nitrogen and crop nitrogen during autumn, winter and spring. J. Agric. Sci. 1987, 109, 141–157. [Google Scholar] [CrossRef]
- Probert, M.E.; Dimes, J.P.; Keating, B.A.; Dalal, R.C.; Strong, W.M. APSIM’s Water and Nitrogen Modules and Simulation of the Dynamics of Water and Nitrogen in Fallow Systems. Agric. Syst. 1998, 56, 1–28. [Google Scholar] [CrossRef]
- Blagodatsky, S.A.; Richter, O. Microbial growth in soil and nitrogen turnover: A theoretical model considering the activity state of microorganisms. Soil Biol. Biochem. 1998, 30, 1743–1755. [Google Scholar] [CrossRef]




| Model | Ref. | Type | Country | Env. | Soil | Climate | Measure Type | n° | Measure Methodology | Simulated Emissions |
|---|---|---|---|---|---|---|---|---|---|---|
| APSIM | [16] | VAL. | China | Arable | Silty–loam | Cfb | Continuous fluxes field measures | 3 | Manual static chambers | Cumulated/ daily |
| [16] | CAL. | China | Arable | Silty–loam | Cfb | Continuous fluxes field measures | 1 | Gas chromatography | Cumulated/ daily | |
| CERES-EGC | [89] | VAL. | Sweden | Arable | Sandy–loam | Cfb | Not-continuous fluxes field measures | 1 | Manual static chambers | Daily |
| [90] | VAL. | Italy | Arable | Clay–loam | Cfa | Continuous fluxes field measures | 1 | Automatic chambers | Daily | |
| CoupModel | [7] | CAL. | Sweden | Lab. experiment | Silty–loam | Cfb | Not-continuos fluxes measures | 16 | Manual static chambers | Cumulated |
| [91] | VAL. | Germany | Arable | Silty–loam | Cfb | Not-continuous fluxes field measures | 3 | Manual static chambers | Daily | |
| DAYCENT | [92] | VAL. | Switzerland | Arable | Silty soil | Cfb | Not-continuous fluxes field measures | 5 | Manual static chambers | Cumulated |
| [11] | VAL. | Colorado | Grassland | Sandy–loam, sandy–clay, clay–loam | Bsk | Not-continuous fluxes field measures | 5 | Automatic chambers | Cumulated/ daily | |
| [93] | VAL. | Colorado | Arable | Sandy–loam, clay–loam | Bsk | Not-continuous fluxes field measures | 2 | Automatic chambers | Cumulated | |
| DNDC | [94] | CAL. | China | Rice/ arable | Clay–loam | Cfa | Not-continuous fluxes field measures | 6 | Manual static chambers | Cumulated/ daily |
| [95] | VAL. | China | Rice/ arable | Silty–clay–loam | Bsk | Continuous fluxes field measures | 3 | Manual static chambers | Cumulated/ daily | |
| EPIC | [96] | CAL. | USA | Arable | Silty–loam | Dfa | Not-continuous fluxes field measures | 7 | Manual static chambers | Cumulated/ daily |
| [96] | VAL. | USA | Arable | Silty–loam | Dfa | Not-continuous fluxes field measures | 7 | Manual static chambers | Cumulated/ daily | |
| [82] | CAL. | USA | Arable | Sandy–loam | Dfa | Not-continuous fluxes field measures | 6 | Manual static chambers | Cumulated/ daily | |
| SPACSYS | [71] | VAL. | Scotland | Grassland | Clay–loam | Cfb | Not-continuous fluxes field measures | 3 | Manual static chambers | Cumulated |
| [97] | VAL. | England | Grassland | Clayey | Cfb | Not-continuous fluxes field measures | 1 | Manual static chambers | Cumulated | |
| [98] | VAL. | England | Grassland | Various | Cfb | Continuous fluxes field measures | 1 | Automatic chambers | Cumulated | |
| [97] | CAL. | England | Grassland | Clayey | Cfb | Not-continuous fluxes field measures | 1 | Manual static chambers | Daily | |
| STICS | [99] | VAL. | Spain Spain–Catalogna France | Arable | Silty–clay–loam (SP), clay–loam (SP-C, FR) | Cfa (SP), Csa (SP-C), Cfb (FR) | Continuous fluxes field measures | 3 | Automatic chambers | Cumulated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabbrielli, M.; Allegrezza, M.; Ragaglini, G.; Manco, A.; Vitale, L.; Perego, A. A Review of the Main Process-Based Approaches for Modeling N2O Emissions from Agricultural Soils. Horticulturae 2024, 10, 98. https://doi.org/10.3390/horticulturae10010098
Gabbrielli M, Allegrezza M, Ragaglini G, Manco A, Vitale L, Perego A. A Review of the Main Process-Based Approaches for Modeling N2O Emissions from Agricultural Soils. Horticulturae. 2024; 10(1):98. https://doi.org/10.3390/horticulturae10010098
Chicago/Turabian StyleGabbrielli, Mara, Marina Allegrezza, Giorgio Ragaglini, Antonio Manco, Luca Vitale, and Alessia Perego. 2024. "A Review of the Main Process-Based Approaches for Modeling N2O Emissions from Agricultural Soils" Horticulturae 10, no. 1: 98. https://doi.org/10.3390/horticulturae10010098
APA StyleGabbrielli, M., Allegrezza, M., Ragaglini, G., Manco, A., Vitale, L., & Perego, A. (2024). A Review of the Main Process-Based Approaches for Modeling N2O Emissions from Agricultural Soils. Horticulturae, 10(1), 98. https://doi.org/10.3390/horticulturae10010098