A Novel Method for Estimating Nitrogen Stress in Plants Using Smartphones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Proof-of-Concept Experiment
2.2. Product Development Experiment
2.3. Experimental Design and Data Analyses
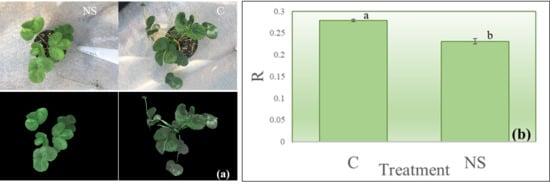
3. Results and Discussion
3.1. Proof-of-Concept Experiment
3.2. Product Development Experiment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Estimation of vegetation water content and photosynthetic tissue area from spectral reflectance: A comparison of indices based on liquid water and chlorophyll absorption features. Remote Sens. Environ. 2003, 84, 526–537. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C 3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Cao, W.; Luo, W.; Dai, T.; Zhu, Y. Monitoring leaf nitrogen status in rice with canopy spectral reflectance. Agron. J. 2004, 96, 135–142. [Google Scholar] [CrossRef]
- Thomas, J.R.; Oerther, G.F. Estimating nitrogen content of sweet pepper leaves by reflectance measurements1. Agron. J. 1972, 64, 11–13. [Google Scholar] [CrossRef]
- Meyer, G.E.; Troyer, W.W.; Fitzgerald, J.B.; Paparozzi, E.T. Leaf nitrogen analysis of poinsettia (Euphorbia pulcherrima Will D.) using spectral properties in natural and controlled lighting. Appl. Eng. Agric. 1992, 8, 715–722. [Google Scholar] [CrossRef]
- Erdle, K.; Mistele, B.; Schmidhalter, U. Comparison of active and passive spectral sensors in discriminating biomass parameters and nitrogen status in wheat cultivars. Field Crop. Res. 2011, 124, 74–84. [Google Scholar] [CrossRef]
- Tewari, V.K.; Arudra, A.K.; Kumar, S.P.; Pandey, V.; Chandel, N.S. Estimation of plant nitrogen content using digital image processing. Agric. Eng. Int. CIGR J. 2013, 15, 78–86. [Google Scholar]
- Kim, Y.; Reid, J.F. Modeling and calibration of a multi-spectral imaging sensor for in-field crop nitrogen assessment. Appl. Eng. Agric. 2006, 22, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Noh, H.; Zhang, Q.; Shin, B.; Han, S.; Feng, L. A neural network model of maize crop nitrogen stress assessment for a multi-spectral imaging sensor. Biosyst. Eng. 2006, 94, 477–485. [Google Scholar] [CrossRef]
- Leemans, V.; Marlier, G.; Destain, M.F.; Dumont, B.; Mercatoris, B. Estimation of leaf nitrogen concentration on winter wheat by multispectral imaging. In Hyperspectral Imaging Sensors: Innovative Applications and Sensor Standards; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; Volume 10213, p. 102130I. [Google Scholar]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Ma, B.L.; Morrison, M.J.; Dwyer, L.M. Canopy light reflectance and field greenness to access nitrogen fertilization and yield of maize. Agric. J. 1996, 88, 915–920. [Google Scholar]
- Baret, F.; Fourty, T. Radiometric estimates of nitrogen status of leaves and canopies. In Diagnosis of the Nitrogen Status in Crops; Springer: Berlin/Heidelberg, Germany, 1997; pp. 201–227. [Google Scholar]
- Zhu, Y.; Yao, X.; Tian, Y.; Liu, X.; Cao, W. Analysis of common canopy vegetation indices for indicating leaf nitrogen accumulations in wheat and rice. Int. J. Appl. Earth Obs. Geoinf. 2008, 10, 1–10. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Ma, B.L.; Belec, C.; Vigneault, P.A. Comparison of crop data measured by two commercial sensors for variable-rate nitrogen application. Precis. Agric. 2009, 10, 145. [Google Scholar] [CrossRef]
- Corti, M.; Gallina, P.M.; Cavalli, D.; Cabassi, G. Hyperspectral imaging of spinach canopy under combined water and nitrogen stress to estimate biomass, water, and nitrogen content. Biosyst. Eng. 2017, 158, 38–50. [Google Scholar] [CrossRef]
- Adhikari, R.; Li, C.; Kalbaugh, K.; Nemali, K. A low-cost smartphone- controlled sensor based on image analysis for estimating whole-plant tissue nitrogen (N) content in floriculture crops. Comput. Electron. Agric. 2020, 169, 105173. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M. Leaf pigment content. In Reference Module in Earth Systems and Environmental Sciences; Elsevier Inc.: Oxford, UK, 2017; pp. 1–22. [Google Scholar]
- Bartley, G.E.; Scolnik, P.A. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. Plant Cell 1995, 7, 1027. [Google Scholar]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral properties of plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Tóth, V.R.; Mészáros, I.; Veres, S.; Nagy, J. Effects of the available nitrogen on the photosynthetic activity and xanthophyll cycle pool of maize in field. J. Plant Physiol. 2002, 159, 627–634. [Google Scholar] [CrossRef]
- Verhoeven, A.S.; Demmig-Adams, B.; Adams, W.W., III. Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and N stress. Plant Physiol. 1997, 113, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Nemali, K.; van Iersel, M.W. Light intensity and fertilizer concentration: II. Optimal fertilizer solution concentration for species differing in light requirement and growth rate. HortScience 2004, 39, 1293–1297. [Google Scholar] [CrossRef] [Green Version]
- Olascoaga, B.; Mac Arthur, A.; Atherton, J.; Porcar-Castell, A. A comparison of methods to estimate photosynthetic light absorption in leaves with contrasting morphology. Tree Physiol. 2016, 36, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.P.; Bernacchi, C.J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 2003, 54, 2393–2401. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Adhikari, R.; Yao, Y.; Miller, A.G.; Kalbaugha, K.; Li, D.; Nemali, K. Measuring plant growth characteristics using smartphone based image analysis technique in controlled environment agriculture. Comput. Electron. Agric. 2020. [Google Scholar] [CrossRef]
- Nemali, K.S.; van Iersel, M.W. An automated system for controlling drought stress and irrigation in potted plants. Sci. Hortic. 2006, 110, 292–297. [Google Scholar] [CrossRef]
- Adhikari, R.; Nemali, K. Substrate versus Fertilizer-based Electrical Conductivity Measurements; HO-322-W; The Education Store; Purdue Extension; Purdue University: West Lafayette, IN, USA, 2020. [Google Scholar]
- Haehnel, W. Photosynthetic electron transport in higher plants. Ann. Rev. Plant Physiol. 1984, 35, 659–693. [Google Scholar] [CrossRef]
- Osborne, B.A.; Raven, J.A. Light absorption by plants and its implications for photosynthesis. Biol. Rev. 1986, 61, 1–6. [Google Scholar] [CrossRef]
- Ding, L.; Wang, K.J.; Jiang, G.M.; Biswas, D.K.; Xu, H.; Li, L.F.; Li, Y.H. Effects of nitrogen deficiency on photosynthetic traits of maize hybrids released in different years. Ann. Bot. 2005, 96, 925–930. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R.; Terashima, I. Effects of nitrogen nutrition on electron transport components and photosynthesis in spinach. Funct. Plant Biol. 1987, 14, 59–68. [Google Scholar] [CrossRef]
- Fredeen, A.L.; Gamon, J.A.; Field, C.B. Responses of photosynthesis and carbohydrate-partitioning to limitations in nitrogen and water availability in field-grown sunflower. Plant Cell Environ. 1991, 14, 963–970. [Google Scholar] [CrossRef]
- Feng, W.; He, L.; Zhang, H.Y.; Guo, B.B.; Zhu, Y.J.; Wang, C.Y.; Guo, T.C. Assessment of plant nitrogen status using chlorophyll fluorescence parameters of the upper leaves in winter wheat. Eur. J. Agron. 2015, 64, 78–87. [Google Scholar] [CrossRef]
- Buschmann, C.; Nagel, E. In vivo spectroscopy and internal optics of leaves as basis for remote sensing of vegetation. Int. J. Remote Sens. 1993, 14, 711–722. [Google Scholar] [CrossRef]
- Mohanty, P.; Boyer, J.S. Chloroplast Response to Low Leaf Water Potentials: IV. Quantum Yield Is Reduced. Plant Physiol. 1976, 57, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Beadle, C.L.; Long, S.P. Photosynthesis—Is it limiting to biomass production? Biomass 1985, 8, 119–168. [Google Scholar] [CrossRef]
- Marcelis, L.F.; Heuvelink, E.; Goudriaan, J. Modelling biomass production and yield of horticultural crops: A review. Sci. Hortic. 1998, 74, 83–111. [Google Scholar] [CrossRef]
- Peng, S.; Krieg, D.R.; Girma, F.S. Leaf photosynthetic rate is correlated with biomass and grain production in grain sorghum lines. Photosynth. Res. 1991, 28, 1–7. [Google Scholar] [CrossRef]
- Wilson, J.W. Analysis of light interception by single plants. Ann. Bot. 1981, 48, 501–505. [Google Scholar] [CrossRef]
- Rosati, A.; Badeck, F.W.; Dejong, T.M. Estimating canopy light interception and absorption using leaf mass per unit leaf area in Solanum melongena. Ann. Bot. 2001, 88, 101–109. [Google Scholar] [CrossRef]






| Treatment | PPFD | ECs | Θ |
|---|---|---|---|
| µmol·m−2·s−1 | dS·m−1 | m3·m−3 | |
| C | 415 (13.4) a | 0.72 (0.027) a | 0.47 (0.016) a |
| NS | 417 (31.0) a | 0.32 (0.017) b | 0.48 (0.013) a |
| DS | 419 (18.8) a | 0.08 (0.014) c | 0.15 (0.01) b |
| Treatment | SDW | CA | A | Iabs | R | ɸPSII |
|---|---|---|---|---|---|---|
| g·plant−1 | m2 | µmol·m−2·s−1 | ||||
| C | 12.2 (0.47) a | 0.20 (0.002) a | 5.2 (0.26) a | 0.95 (0.008) a | 0.296 (0.008) b | 0.31 (0.018) a |
| NS | 10.0 (0.69) b | 0.19 (0.002) a | 3.8 (0.56) b | 0.95 (0.013) a | 0.271 (0.021) a | 0.26 (0.005) a |
| DS | 6.0 (0.78) c | 0.14 (0.008) b | 1.8 (0.27) c | 0.92 (0.003) b | 0.292 (0.006) b | 0.28 (0.022) a |
| Treatment | Gray Value (0 to 255 Scale) | |||
|---|---|---|---|---|
| Petunia | Strawberry | |||
| 450 nm | (450 + 521 + 660) nm | blue | (blue + green + red) | |
| C | 74.2 (1.18) a | 253.3 (3.87) b | 77.4 (3.90) a | 277.1 (12.26) b |
| NS | 76.4 (1.22) a | 267.6 (3.94) a | 73.2 (1.68) a | 317.0 (5.83) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, R.; Nemali, K. A Novel Method for Estimating Nitrogen Stress in Plants Using Smartphones. Horticulturae 2020, 6, 74. https://doi.org/10.3390/horticulturae6040074
Adhikari R, Nemali K. A Novel Method for Estimating Nitrogen Stress in Plants Using Smartphones. Horticulturae. 2020; 6(4):74. https://doi.org/10.3390/horticulturae6040074
Chicago/Turabian StyleAdhikari, Ranjeeta, and Krishna Nemali. 2020. "A Novel Method for Estimating Nitrogen Stress in Plants Using Smartphones" Horticulturae 6, no. 4: 74. https://doi.org/10.3390/horticulturae6040074
APA StyleAdhikari, R., & Nemali, K. (2020). A Novel Method for Estimating Nitrogen Stress in Plants Using Smartphones. Horticulturae, 6(4), 74. https://doi.org/10.3390/horticulturae6040074