Ethephon-Induced Abscission of Oil Palm Fruits at Optimal Bunch Ripeness and Retting Period to Improve Commercial Seed Production
Abstract
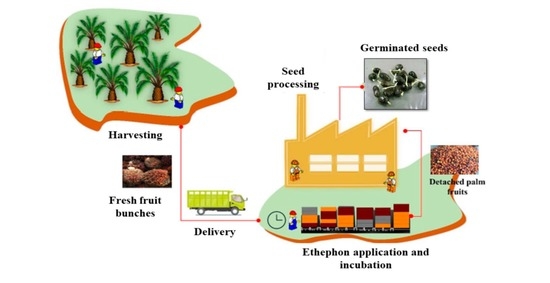
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Ethephon Treatment, Quantification of Loose Fruits and Retting Period
2.3. Ethephon Effect on Seed Germination and Culling Rate for 145 DAP
2.4. Statistical Analysis
3. Results
3.1. Effect of FFB Ripeness on Fruit Abscission upon Harvesting
3.2. Effect of Ethephon Treatment on Fruit Abscission after 72-h Incubation and Retting Period across FFB Ripeness
3.3. Effect of Posttreatment with Ethephon on Seed Germination and Culling at Nursery for 145 DAP
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meijaard, E.; Garcia-Ulloa, J.; Sheil, D.; Wich, S.A.; Carlson, K.M.; Juffe-Bignoli, D.; Brooks, T.M. Oil Palm and Biodiversity: A Situation Analysis by the IUCN Oil Palm Task Force, 1st ed.; International Union for Conservation of Nature and Natural Resources (IUCN): Gland, Switzerland, 2018. [Google Scholar] [CrossRef]
- Ghulam Kadir, A.P. Oil palm economic performance in Malaysia and R&D progress in 2019. J. Oil Palm Res. 2020, 32, 159–190. [Google Scholar] [CrossRef]
- Corley, R.H.V.; Tinker, P.B. The Origin and Development of the Oil Palm Industry. In The Oil Palm; Corley, R.H.V., Tinker, P.B., Eds.; Wiley Blackwell: Oxford, UK, 2015; pp. 1–29. [Google Scholar] [CrossRef]
- Hardon, J.J.; Corley, R.H.V.; Lee, C.H. Breeding and selecting the oil palm. In Improving Vegetatively Propagated Crops; Abbot, A.J., Atkin, R.K., Eds.; Academic Press: London, UK, 1987; pp. 63–81. [Google Scholar]
- Choong, C.G.; McKay, A. Sustainability in the Malaysian palm oil industry. J. Clean. Prod. 2014, 85, 258–264. [Google Scholar] [CrossRef]
- Henry Ezechi, E.; Muda, K. Overview of trends in crude palm oil production and economic impact in Malaysia. Sriwij. J. Environ. 2019, 4, 19–26. [Google Scholar] [CrossRef]
- Wong, E.L.; The EdgeTM Markets. MPOC: Palm Oil Production to Increase to 19.6 Million Tonnes in 2021. 2021. Available online: https://www.theedgemarkets.com/article/mpoc-palm-oil-production-increase-196-million-tonnes-2021 (accessed on 1 June 2021).
- Abdullah, R. World palm oil supply, demand, price and prospects: Focus on Malaysian and Indonesian Palm Oil Industries. Oil Palm Ind. Econ. J. 2011, 11, 13–25. [Google Scholar]
- Rajanaidu, N.; Ainul, M.M.; Kushairi, A.; Din, A. Historical review of oil palm breeding for the past 50 years—Malaysian Journey. In Proceedings of the International Seminar on Oil Palm Breeding—Yesterday, Today and Tomorrow, Kuala Lumpur, Malaysia, 18 November 2013; pp. 11–28. [Google Scholar]
- Teh, C.-K.; Global Engage. Is Palm Oil Sustainable for People? 2018. Available online: https://www.global-engage.com/agricultural-biotechnology/is-palm-oil-sustainable-for-people/ (accessed on 7 September 2021).
- Burdon, J.N.; Sexton, R. The Role of Ethylene in the Shedding of Red Raspberry Fruit. Ann. Bot. 1990, 66, 111–120. [Google Scholar] [CrossRef]
- Eccher, G.; Begheldo, M.; Boschetti, A.; Ruperti, B.; Botton, A. Roles of Ethylene Production and Ethylene Receptor Expression in Regulating Apple Fruitlet Abscission. Plant Physiol. 2015, 169, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Mohanaraj, S.N.; Donough, C.R. Harvesting practices for maximum yield in oil palm: Results from a re-assessment at IJM Plantations, Sabah. Oil Palm Bull. 2016, 72, 32–37. [Google Scholar]
- Mora, S.; Chinchilla, C.; Sánchez, A.; Escobar, R. Germinated oil palm (Elaeis guineensis) seeds: Process innovations to improve seed quality and performance of nursery plants. Planter 2007, 83, 435–446. [Google Scholar]
- Al-Saif, A.; Alebidi, A.I.; Alobeed, R.; Soliman, S.S. Preharvest Ethephon spray on fruit quality and increasing the rate of ripening of date palm fruit (Phoenix dactylifera L.) cv. Helali. Prog. Nutr. 2017, 19, 97–103. [Google Scholar] [CrossRef]
- Rouhani, I.; Bassiri, A. Effect of Ethephon on Ripening and Physiology of Date Fruits at Different Stages of Maturity. J. Hortic. Sci. 2015, 52, 289–297. [Google Scholar] [CrossRef]
- Juntaraniyom, T.; Tongkum, P.; Eksomtramage, T.; Chuntanalurg, O. Use of calcium carbide and ethephon to stimulate fruit drop of oil palm. Songklanakarin J. Sci. Technol. 1996, 18, 293–299. [Google Scholar]
- Nualwijit, N.; Lerslerwong, L. Post harvest ripening of oil palm fruit is accelerated by application of exogenous ethylene. Songklanakarin J. Sci. Technol. 2014, 36, 255–259. [Google Scholar]
- Nurniwalis, A.W.; Zubaidah, R.; Siti Nor, A.A.; Suhaimi, N.; Massawe, F. Isolation and Characterisation of an ethylene receptor (ERS-Type) from Oil Palm (Elaeis guineensis Jacq.) Mesocarp. J. Oil Palm Res. 2018, 30, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Tranbarger, T.J.; Dussert, S.; Joët, T.; Argout, X.; Summo, M.; Champion, A.; Cros, D.; Omore, A.; Nouy, B.; Morcillo, F. Regulatory Mechanisms Underlying Oil Palm Fruit Mesocarp Maturation, Ripening, and Functional Specialization in Lipid and Carotenoid Metabolism. Plant Physiol. 2011, 156, 564–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mat Hassan, N.S.; Kua, S.F.; Noor Haizat, A.H.; Mustaner, M.; Mohd Hakimi, N.I.N.; Syed Hilmi, S.M.H.; Balakrishnan, A.; Tan, B.A.; Lim, C.M.; Jaime Low, Y.S.; et al. Process for Producing Crude Palm Fruit Oil and Virgin Palm Fruit Oil; Intellectual Property Corporation of Malaysia: Kuala Lumpur, Malaysia, 2021.
- Malaysian Standard. MS 157:2005 Oil Palm Seeds for Commercial Planting—Specification (Third Revision); Department of Standards Malaysia: Selangor, Malaysia, 2005.
- Minitab 20 Statistical Software; Minitab Inc.: State College, PA, USA, 2020.
- Soh, A.C.; Mayes, S.; Roberts, J.; Zaki, N.M.; Madon, M.; Schwarzacher, T.; Heslop-Harrison, P.; Ithnin, M.; Amiruddin, M.D.; Ramli, U.S.; et al. Molecular Genetics and Breeding. In Oil Palm Breeding: Genetics and Genomics; Aik, C.S., Mayes, S., Roberts, J., Eds.; CRC Press: Atlanta, FL, USA, 2017; pp. 225–281. [Google Scholar]
- Bernama. Sime Darby Plantation: ‘Genomeselect’ Oil Palm Seeds Boost Yields on Existing Land. Malay Mail. 21 July 2020. Available online: https://www.malaymail.com/news/money/2020/07/21/sime-darby-plantation-genomeselect-oil-palm-seeds-boost-yields-on-existing/1886671 (accessed on 10 December 2020).
- Shankar, A.C.; The Edge Markets. Sime Darby Plantation Plans to Fully Use GenomeSelect Seeds for Oil Palm Replanting from 2023. 2020. Available online: https://www.theedgemarkets.com/article/sime-darby-plantation-plans-fully-use-genomeselect-seeds-oil-palm-replanting-2023 (accessed on 20 August 2020).
- Roongsattham, P.; Morcillo, F.; Jantasuriyarat, C.; Pizot, M.; Moussu, S.; Jayaweera, D.; Collin, M.; Gonzalez-Carranza, Z.H.; Amblard, P.; Tregear, J.W.; et al. Temporal and spatial expression of polygalacturonase gene family members reveals divergent regulation during fleshy fruit ripening and abscission in the monocot species oil palm. BMC Plant Biol. 2012, 12, 150. [Google Scholar] [CrossRef] [Green Version]
- Chalil, D.; Basyuni, M.; Barus, R.; Putri, L.A. Smallholders’ willingness to pay for dura marking oil palm seeds. E3S Web Conf. 2018, 52, 11. [Google Scholar] [CrossRef]
- Singh, R.; Low, E.T.; Ooi, L.C.; Ong-Abdullah, M.; Ting, N.C.; Nagappan, J.; Nookiah, R.; Amiruddin, M.D.; Rosli, R.; Manaf, M.A.; et al. The oil palm SHELL gene controls oil yield and encodes a homologue of SEEDSTICK. Nature 2013, 500, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Teh, C.-K.; Lee, H.-L.; Abidin, H.; Ong, A.-L.; Mayes, S.; Chew, F.-T.; Appleton, D. A practical genome-enabled legitimacy assay for oil palm breeding and seed production. BMC Plant Biol. 2019, 19, 470. [Google Scholar] [CrossRef] [Green Version]
- Dikin, A.; Kamaruzaman, S.; Zainal Abidin, M.A.; Idris Abu, S. Biological control of seedborne pathogen of oil palm, Schizopyllum commune Fr. with antagonistic bacteria. Int. J. Agric. Biol. 2003, 5, 507–512. [Google Scholar]
- Pongprasert, N.; Srilaong, V.; Sugaya, S. An alternative technique using ethylene micro-bubble technology to accelerate the ripening of banana fruit. Sci. Hortic. 2020, 272, 109566. [Google Scholar] [CrossRef]
- Wu, Q.; Bai, J.; Tao, X.; Mou, W.; Luo, Z.; Mao, L.; Ban, Z.; Ying, T.; Li, L. Synergistic effect of abscisic acid and ethylene on color development in tomato (Solanum lycopersicum L.) fruit. Sci. Hortic. 2018, 235, 169–180. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, M.; Zhang, J.; Ge, Y.; Li, C.; Meng, K.; Li, J. Effects of methyl jasmonate on expression of genes involved in ethylene biosynthesis and signaling pathway during postharvest ripening of apple fruit. Sci. Hortic. 2018, 229, 157–166. [Google Scholar] [CrossRef]
- Kou, J.; Wei, C.; Zhao, Z.; Guan, J.; Wang, W. Effects of ethylene and 1-methylcyclopropene treatments on physiological changes and ripening-related gene expression of ‘Mopan’ persimmon fruit during storage. Postharvest Biol. Technol. 2020, 166, 111185. [Google Scholar] [CrossRef]
- Suryanto, H.; Bardaie, M.Z. Effective treatment to hasten oil palm fruitlets abscission using ethephon. AMA Agric. Mech. Asia Afr. Lat. Am. 1994, 25, 40–44. [Google Scholar]
- Ismail, W.I.W.; Yip, L.W.; Razali, M.H. Determination of the optimum frequency for Elaeis guineensis Jacq. detachment. Afr. J. Agric. Res. 2011, 6, 5656–5663. [Google Scholar]
- Herrera, J.; Alizaga, R.; Guevara, E. Use of chemical treatments to induce seed germination in oil palm Elaeis guineensis Jacq. ASD Oil Palm Pap. 1998, 18, 1–16. [Google Scholar]
- Bicalho, E.M.; Pintó-Marijuan, M.; Morales, M.; Müller, M.; Munné-Bosch, S.; Garcia, Q.S. Control of macaw palm seed germination by the gibberellin/abscisic acid balance. Plant Biol. 2015, 17, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Vanangamudi, K.; Zope, J.S.; Elam, W.W. Effect of Ethephon on Dormancy and Germination of Loblolly Pine (Pinus taeda) seed. J. Trop. For. Sci. 1999, 11, 503–506. [Google Scholar]
- Heriansyah, C.; Tan, C. Nursery practices for production of superior oil palm planting materials. Planter 2005, 81, 159–171. [Google Scholar]
- Wan, C.K.; Hor, H.L. A Study on the Effects of Certain Growth Substances on Germination of Oil Palm (Elaeis quineensis Jacq.) Seeds. Pertanika 1983, 6, 45–48. [Google Scholar]
- Laksono, N.D.; Setiawati, U.; Nur, F.; Rahmaningsih, M.; Anwar, Y.; Rusfiandi, H.; Sembiring, E.H.; Forster, B.; Subbarao, A.S.; Zahara, H. Nursery Practices in Oil Palm: A Manual; CABI: Wallingford, UK, 2019; p. 120. [Google Scholar]
- Mathews, J.; Tan, T.H.; Yong, K.K.; Chong, K.M.; Ng, S.K.; IP, W.M. Managing oil palm nursery: IOI’s experience. Planter 2010, 86, 771–785. [Google Scholar]
- MPOB. Code of Good Nursery Practice for Oil Palm Nurseries; MPOB CoP 1001:2015; MPOB: Kajang, Malaysia, 2016.
- Sharma, M.; Vijiandran, J.; Applanaidu, M. Oil palm nursery management—A perspective from UP Bhd. In Proceedings of the International Seminar on Oil Planting Materials for Local & Overseas Joint Ventures, Hotel Istana, Asgard Information Services. Kuala Lumpur, Malaysia, December 2007. [Google Scholar]
- Corley, R.H.V.; Tinker, P.B. Seed Germination and Nurseries. In The Oil Palm; Corley, R.H.V., Tinker, P.B., Eds.; Wiley Blackwell: Oxford, UK, 2016; p. 674. [Google Scholar]




| Days after Pollination (DAP) | Number of FFB | |
|---|---|---|
| Control | Ethephon-Treated | |
| 125 | 9 | 10 |
| 135 | 6 | 8 |
| 145 | 6 | 6 |
| 150 | 7 | 8 |
| 160 | 3 | 3 |
| Total | 31 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, J.Y.-S.; Fong, P.-Y.; Teh, C.-K.; Ong, A.-L.; Lim, C.-M.; Appleton, D.R. Ethephon-Induced Abscission of Oil Palm Fruits at Optimal Bunch Ripeness and Retting Period to Improve Commercial Seed Production. Horticulturae 2021, 7, 380. https://doi.org/10.3390/horticulturae7100380
Low JY-S, Fong P-Y, Teh C-K, Ong A-L, Lim C-M, Appleton DR. Ethephon-Induced Abscission of Oil Palm Fruits at Optimal Bunch Ripeness and Retting Period to Improve Commercial Seed Production. Horticulturae. 2021; 7(10):380. https://doi.org/10.3390/horticulturae7100380
Chicago/Turabian StyleLow, Jaime Yoke-Sum, Po-Yee Fong, Chee-Keng Teh, Ai-Ling Ong, Chin-Ming Lim, and David Ross Appleton. 2021. "Ethephon-Induced Abscission of Oil Palm Fruits at Optimal Bunch Ripeness and Retting Period to Improve Commercial Seed Production" Horticulturae 7, no. 10: 380. https://doi.org/10.3390/horticulturae7100380
APA StyleLow, J. Y. -S., Fong, P. -Y., Teh, C. -K., Ong, A. -L., Lim, C. -M., & Appleton, D. R. (2021). Ethephon-Induced Abscission of Oil Palm Fruits at Optimal Bunch Ripeness and Retting Period to Improve Commercial Seed Production. Horticulturae, 7(10), 380. https://doi.org/10.3390/horticulturae7100380