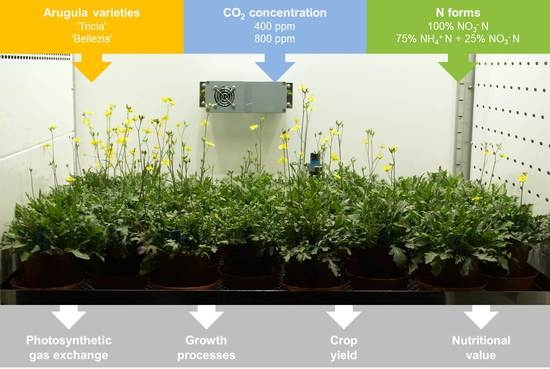
For a Better Understanding of the Effect of N Form on Growth and Chemical Composition of C3 Vascular Plants under Elevated CO2—A Case Study with the Leafy Vegetable Eruca sativa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Biomass and Physiological Measurements
2.3. Chemical Analyses
2.4. Statistical Analyses
3. Results
3.1. Substrate Composition
3.2. Plant Growth and Yield Parameters
3.3. Photosynthetic Gas Exchange
3.4. Plant Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Global Warming of 1.5 °C: An IPCC Special Report On the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. 2018. Available online: www.ipcc.ch/sr15/ (accessed on 7 July 2021).
- Gamage, D.; Thompson, M.; Sutherland, M.; Hirotsu, N.; Makino, A.; Seneweera, S. New insights into the cellular mechanisms of plant growth at elevated atmospheric carbon dioxide concentrations. Plant Cell Environ. 2018, 41, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising CO2: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J. Photorespiration and nitrate assimilation: A major intersection between plant carbon and nitrogen. Photosynth. Res. 2015, 123, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Stefanelli, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Rubio-Asensio, J.S.; Bloom, A.J. Inorganic nitrogen form: A major player in wheat and Arabidopsis responses to elevated CO2. J. Exp. Bot. 2017, 68, 2611–2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galmés, J.; Flexas, J.; Keys, A.J.; Cifre, J.; Mitchell, R.A.C.; Madgwick, P.J.; Haslam, R.P.; Medrano, H.; Parry, M.A.J. Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ. 2005, 28, 571–579. [Google Scholar] [CrossRef]
- Körner, C. Plant CO2 responses: An issue of definition, time and resource supply. New Phytol. 2006, 172, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Asensio, J.S.R.; Randall, L.; Rachmilevitch, S.; Cousins, A.B.; Carlisle, E.A. CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 2012, 93, 355–367. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Rubio Asensio, J.S.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Andrews, M.; Condron, L.M.; Kemp, P.D.; Topping, J.F.; Lindsey, K.; Hodge, S.; Raven, J.A. Elevated CO2 effects on nitrogen assimilation and growth of C3 vascular plants are similar regardless of N-form assimilated. J. Exp. Bot. 2019, 70, 683–690. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Walch-Liu, P.; Neumann, G.; Bangerth, F.; Engels, C. Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J. Exp. Bot. 2000, 51, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Hachiya, T.; Watanabe, C.K.; Fujimoto, M.; Ishikawa, T.; Takahara, K.; Kawai-Yamada, M.; Uchimiya, H.; Uesono, Y.; Terashima, I.; Noguchi, K. Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana shoots. Plant Cell Physiol. 2012, 53, 577–591. [Google Scholar] [CrossRef]
- Torralbo, F.; González-Moro, M.B.; Baroja-Fernández, E.; Aranjuelo, I.; González-Murua, C. Differential regulation of stomatal conductance as a strategy to cope with ammonium fertilizer under ambient versus elevated CO2. Front. Plant Sci. 2019, 10, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, M.; Condron, L.M.; Kemp, P.D.; Topping, J.F.; Lindsey, K.; Hodge, S.; Raven, J.A. Will rising atmospheric CO2 concentration inhibit nitrate assimilation in shoots but enhance it in roots of C3 plants? Physiol. Plant 2020, 170, 40–45. [Google Scholar] [CrossRef]
- Rowland-Bamford, A.J.; Baker, J.T.; Allen, L.H.J.; Bowes, G. Acclimation of rice to changing atmospheric carbon dioxide concentration. Plant Cell Environ. 1991, 14, 577–583. [Google Scholar] [CrossRef]
- Theobald, J.C.; Mitchell, R.A.C.; Parry, M.A.J.; Lawlor, D.W. Estimating the excess investment in ribulose-1,5-bisphosphate carboxylase/oxygenase in leaves of spring wheat grown under elevated CO2. Plant Physiol. 1998, 118, 945–955. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.D.; Cheng, S.H.; Sims, D.; Seemann, J.R. The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ. 1999, 22, 567–582. [Google Scholar] [CrossRef]
- Geiger, M.; Haake, V.; Ludewig, F.; Sonnewald, U.; Stitt, M. The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant Cell Environ. 1999, 22, 1177–1199. [Google Scholar] [CrossRef]
- Matt, P.; Geiger, M.; Walch-Liu, P.; Engels, C.; Krapp, A.; Stitt, M. Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant Cell Environ. 2001, 24, 1119–1137. [Google Scholar] [CrossRef]
- Dong, J.; Li, X.; Chu, W.; Duan, Z. High nitrate supply promotes nitrate assimilation and alleviates photosynthetic acclimation of cucumber plants under elevated CO2. Sci. Hortic. 2017, 218, 275–283. [Google Scholar] [CrossRef]
- Bloom, A.J.; Kasemsap, P.; Rubio-Asensio, J.S. Rising atmospheric CO2 concentration inhibits nitrate assimilation in shoots but enhances it in roots of C3 plants. Physiol. Plant 2020, 168, 963–972. [Google Scholar] [CrossRef]
- Kim, S.-J.; Chiami, K.; Ishii, G. Effect of ammonium: Nitrate nutrient ratio on nitrate and glucosinolate contents of hydroponically-grown rocket salad (Eruca sativa Mill.). Soil Sci. Plant Nutr. 2006, 52, 387–393. [Google Scholar] [CrossRef]
- Albornoz, F. Crop responses to nitrogen overfertilization: A review. Sci. Hortic. 2016, 205, 79–83. [Google Scholar] [CrossRef]
- Shang, H.Q.; Shen, G.M. Effect of ammonium/nitrate ratio on pak choi (Brassica chinensis L.) photosynthetic capacity and biomass accumulation under low light intensity and water deficit. Photosynthetica 2018, 56, 1039–1046. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Papa, G.; Serio, F. Nitrate and ammonium nutrition in chicory and rocket salad plants. J. Plant Nutr. 1998, 21, 1779–1789. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Parente, A.; Serio, F. Fertilization strategies for lowering nitrate content in leafy vegetables: Chicory and rocket salad cases. J. Plant Nutr. 1998, 21, 1791–1803. [Google Scholar] [CrossRef]
- Zaghdoud, C.; Carvajal, M.; Moreno, D.A.; Ferchichi, A.; Del Carmen Martínez-Ballesta, M. Health-promoting compounds of broccoli (Brassica oleracea L. var. italica) plants as affected by nitrogen fertilisation in projected future climatic change environments. J. Sci. Food Agric. 2016, 96, 392–403. [Google Scholar] [CrossRef]
- Marino, D.; Ariz, I.; Lasa, B.; Santamaria, E.; Fernandez-Irigoyen, J.; Gonzalez-Murua, C.; Aparicio Tejo, P.M. Quantitative proteomics reveals the importance of nitrogen source to control glucosinolate metabolism in Arabidopsis thaliana and Brassica oleracea. J. Exp. Bot. 2016, 67, 3313–3323. [Google Scholar] [CrossRef] [Green Version]
- Chitarra, W.; Siciliano, I.; Ferrocino, I.; Gullino, M.L.; Garibaldi, A. Effect of elevated atmospheric CO2 and temperature on the disease severity of rocket plants caused by fusarium wilt under phytotron conditions. PLoS ONE 2015, 10, e0140769. [Google Scholar] [CrossRef] [Green Version]
- Gilardi, G.; Pugliese, M.; Chitarra, W.; Ramon, I.; Gullino, M.L.; Garibaldi, A. Effect of elevated atmospheric CO2 and temperature increases on the severity of basil downy mildew caused by Peronospora belbahrii under phytotron conditions. J. Phytopathol. 2016, 164, 114–121. [Google Scholar] [CrossRef]
- Eastburn, D.M.; McElrone, A.J.; Bilgin, D.D. Influence of atmospheric and climatic change on plant-pathogen interactions. Plant Pathol. 2011, 60, 54–69. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, M.; Hwang, J.E.; Kim, S.H.; Kim, J.A.; Jeong, M.-J.; Park, H.C.; Lee, S.I. Elevated carbon dioxide significantly improves ascorbic acid content, antioxidative properties and restricted biomass production in cruciferous vegetable seedlings. Plant Biotechnol. Rep. 2019, 13, 293–304. [Google Scholar] [CrossRef]
- Jain, V.; Pal, M.; Raj, A.; Khetarpal, S. Photosynthesis and nutrient composition of spinach and fenugreek grown under elevated carbon dioxide concentration. Biol. Plant 2007, 51, 559–562. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. elife 2014, 3, e02245. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Jobling, J.; Rogers, G. Some perspectives on rocket as a vegetable crop: A review. Veg. Crop. Res. Bull. 2012, 76, 334. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Rincón-Cervera, M.A.; González-Fernández, M.J.; Guil-Guerrero, J.L. Phytochemical composition and antitumor activities of new salad greens: Rucola (Diplotaxis tenuifolia) and corn salad (Valerianella locusta). Plant Foods Hum. Nutr. 2016, 71, 197–203. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef] [Green Version]
- Weinheimer, S.; Naab, B. Deutliche Unterschiede bei Rucola hinsichtlich der Anfälligkeit bei Falschem Mehltau und dem Schossverhalten. In Versuche im Deutschen Gartenbau—Ökologischer Gemüsebau; Verband der Landwirtschaftskammern e.V.: Berlin, Germany, 2014; pp. 230–232. (In German) [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. Available online: www.imagej.net/software/fiji/ (accessed on 7 July 2021). [CrossRef] [PubMed] [Green Version]
- Ensminger, I.; Xyländer, M.; Hagen, C.; Braune, W. Strategies providing success in a variable habitat: III. Dynamic control of photosynthesis in Cladophora glomerata. Plant Cell Environ. 2001, 24, 769–779. [Google Scholar] [CrossRef]
- Mancinelli, A.L. Interaction between light quality and light quantitiy in the photoregulation of anthocyanin production. Plant Physiol. 1990, 92, 1191–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, J.R.; Haby, V.A. Simplified colorimetric determination of soil organic matter. Soil Sci. 1971, 112, 137–141. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: www.R-project.org (accessed on 7 July 2021).
- Bloom, A.J. Rising carbon dioxide concentrations and the future of crop production. J. Sci. Food Agric. 2006, 86, 1289–1291. [Google Scholar] [CrossRef]
- Van Oosten, J.J.; Besford, R.T. Acclimation of photosynthesis to elevated CO2 through feedback regulation of gene expression: Climate of opinion. Photosynth. Res. 1996, 48, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Cazzonelli, C.I. Short photoperiod attenuates CO2 fertilization effect on shoot biomass in Arabidopsis thaliana. Physiol. Mol. Biol. Plants 2021, 27, 825–834. [Google Scholar] [CrossRef]
- Tausz-Posch, S.; Tausz, M.; Bourgault, M. Elevated CO2 effects on crops: Advances in understanding acclimation, nitrogen dynamics and interactions with drought and other organisms. Plant Biol. 2020, 22 (Suppl. 1), 38–51. [Google Scholar] [CrossRef]
- Jauregui, I.; Aparicio-Tejo, P.M.; Avila, C.; Cañas, R.; Sakalauskiene, S.; Aranjuelo, I. Root-shoot interactions explain the reduction of leaf mineral content in Arabidopsis plants grown under elevated CO2 conditions. Physiol. Plant. 2016, 158, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Zinkernagel, J. How to optimize the cultivation of arugula under elevated atmospheric CO2 and different nitrogen forms? DGG Proc. 2018, 8, 1–5. [Google Scholar]
- Jin, C.W.; Du, S.T.; Zhang, Y.S.; Tang, C.; Lin, X.Y. Atmospheric nitric oxide stimulates plant growth and improves the quality of spinach (Spinacia oleracea ). Ann. Appl. Biol. 2009, 155, 113–120. [Google Scholar] [CrossRef]
- Dong, A.; Armstrong, B.; Rajashekar, C.B. Elevated carbon dioxide level suppresses nutritional quality of lettuce and spinach. Am. J. Plant Sci. 2016, 7, 246–258. [Google Scholar] [CrossRef] [Green Version]
- Becker, C.; Kläring, H.-P. CO2 enrichment can produce high red leaf lettuce yield while increasing most flavonoid glycoside and some caffeic acid derivative concentrations. Food Chem. 2016, 199, 736–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescott, C.E.; Grayston, S.J.; Helmisaari, H.-S.; Kaštovská, E.; Körner, C.; Lambers, H.; Meier, I.C.; Millard, P.; Ostonen, I. Surplus carbon drives allocation and plant-soil interactions. Trends Ecol. Evol. 2020, 35, 1110–1118. [Google Scholar] [CrossRef]
- Loladze, I. Rising atmospheric CO2 and human nutrition: Toward globally imbalanced plant stoichiometry? Trends Ecol. Evol. 2002, 17, 457–461. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef]
- Sato, S.; Yanagisawa, S. Characterization of metabolic states of Arabidopsis thaliana under diverse carbon and nitrogen nutrient conditions via targeted metabolomic analysis. Plant Cell Physiol. 2014, 55, 306–319. [Google Scholar] [CrossRef] [Green Version]



| Treatment | Total N (mg·L−1) | NH4+-N (mg·L−1) | NO3−-N (mg·L−1) | pH |
|---|---|---|---|---|
| aBeNO | 65.6 a | 30.1 a | 35.5 a | 7.0 a |
| aTriNO | 62.6 a | 15.6 a | 47.0 a | 7.0 a |
| aBeNH | 83.1 a | 28.7 a | 54.4 a | 6.1 b |
| aTriNH | 63.4 a | 27.7 a | 35.7 a | 5.8 c |
| eBeNO | 60.1 a | 23.4 a | 36.7 a | 7.1 a |
| eTriNO | 67.7 a | 20.1 a | 47.5 a | 7.0 a |
| eBeNH | 84.0 a | 31.4 a | 52.6 a | 6.1 b |
| eTriNH | 56.1 a | 24.7 a | 31.5 a | 5.6 c |
| Treatment | Fresh Mass (g·plant−1) | Dry Mass (g·plant−1) | Leaf Number (plant−1) | Total Leaf Area (cm2·plant−1) | Inflorescences (Number pot−1) |
|---|---|---|---|---|---|
| aBeNO | 1.53 a | 0.27 ac | 8.11 a | 25.48 ab | 4.7 c |
| aTriNO | 1.64 a | 0.28 ad | 7.81 a | 30.10 c | 4.1 c |
| aBeNH | 1.11 b | 0.18 b | 8.09 a | 21.25 de | 2.6 d |
| aTriNH | 1.28 ce | 0.22 ab | 7.70 a | 25.84 ab | 3.7 c |
| eBeNO | 1.59 ad | 0.32 cd | 8.52 a | 25.66 ab | 6.5 ac |
| eTriNO | 1.66 a | 0.32 cd | 8.20 a | 28.30 ac | 7.4 ac |
| eBeNH | 1.14 bc | 0.23 ab | 8.46 a | 19.04 d | 5.8 b |
| eTriNH | 1.30 e | 0.26 a | 8.19 a | 24.03 be | 5.9 b |
| Treatment | Chlorophylls (mg 100 g−1 FM) | Carotenoids (mg 100 g−1 FM) | Anthocyanins (mg 100 g−1 FM) | Ascorbic Acid (mg 100 g−1 FM) |
|---|---|---|---|---|
| aBeNO | 30.5 a | 0.25 a | 8.3 a | 24.5 ab |
| aTriNO | 29.8 a | 0.16 a | 8.1 a | 18.1 a |
| aBeNH | 37.5 a | 0.18 a | 9.4 a | 18.1 a |
| aTriNH | 29.1 a | 0.15 a | 6.8 a | 21.5 ab |
| eBeNO | 29.7 a | 0.49 a | 5.5 a | 24.8 ab |
| eTriNO | 25.5 a | 0.40 a | 6.2 a | 21.8 ab |
| eBeNH | 30.3 a | 0.25 a | 5.7 a | 26.6 ab |
| eTriNH | 30.1 a | 0.32 a | 6.4 a | 35.2 b |
| Element | Unit | aBeNO | aTriNO | aBeNH | aTriNH | eBeNO | eTriNO | eBeNH | eTriNH |
|---|---|---|---|---|---|---|---|---|---|
| C | % | 39.1 a | 37.8 a | 38.1 a | 38.6 a | 39.2 a | 39.5 a | 39.9 a | 39.6 a |
| N | % | 2.19 a | 2.16 a | 2.97 a | 2.47 a | 1.92 a | 1.85 a | 2.61 a | 2.51 a |
| S | % | 0.96 ab | 1.12 abc | 1.31 b | 1.20 ab | 0.81 c | 0.90 c | 1.02 abc | 0.94 ac |
| K | % | 2.91 bc | 3.18 bc | 3.17 bc | 3.30 b | 2.68 ac | 2.73 ac | 2.94 bc | 3.01 bc |
| P | % | 0.39 a | 0.36 a | 0.71 b | 0.61 bc | 0.33 a | 0.33 a | 0.63 bc | 0.57 c |
| Ca | % | 1.87 a | 1.97 a | 1.65 a | 1.37 a | 1.73 a | 1.74 a | 1.38 a | 1.33 a |
| Mg | % | 0.22 a | 0.24 a | 0.22 a | 0.22 a | 0.21 a | 0.22 a | 0.21 a | 0.21 a |
| Fe | ppm | 41.4 a | 42.7 a | 60.1 a | 71.5 a | 39.4 a | 39.1 a | 71.9 a | 63.6 a |
| Zn | ppm | 20.3 a | 21.0 abd | 28.3 bc | 30.9 c | 20.5 ab | 20.9 abd | 31.1 c | 28.3 cd |
| Mn | ppm | 11.4 a | 10.7 a | 12.5 a | 18.4 a | 11.0 a | 9.1 a | 13.7 a | 16.7 a |
| Cu | ppm | 6.44 a | 7.04 a | 8.09 a | 6.87 a | 7.34 a | 6.47 a | 7.19 a | 6.84 a |
| Na | ppm | 0.16 ab | 0.15 ab | 0.22 a | 0.15 ab | 0.13 ab | 0.13 b | 0.15 ab | 0.13 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, L.; Zinkernagel, J. For a Better Understanding of the Effect of N Form on Growth and Chemical Composition of C3 Vascular Plants under Elevated CO2—A Case Study with the Leafy Vegetable Eruca sativa. Horticulturae 2021, 7, 251. https://doi.org/10.3390/horticulturae7080251
Schmidt L, Zinkernagel J. For a Better Understanding of the Effect of N Form on Growth and Chemical Composition of C3 Vascular Plants under Elevated CO2—A Case Study with the Leafy Vegetable Eruca sativa. Horticulturae. 2021; 7(8):251. https://doi.org/10.3390/horticulturae7080251
Chicago/Turabian StyleSchmidt, Lilian, and Jana Zinkernagel. 2021. "For a Better Understanding of the Effect of N Form on Growth and Chemical Composition of C3 Vascular Plants under Elevated CO2—A Case Study with the Leafy Vegetable Eruca sativa" Horticulturae 7, no. 8: 251. https://doi.org/10.3390/horticulturae7080251
APA StyleSchmidt, L., & Zinkernagel, J. (2021). For a Better Understanding of the Effect of N Form on Growth and Chemical Composition of C3 Vascular Plants under Elevated CO2—A Case Study with the Leafy Vegetable Eruca sativa. Horticulturae, 7(8), 251. https://doi.org/10.3390/horticulturae7080251