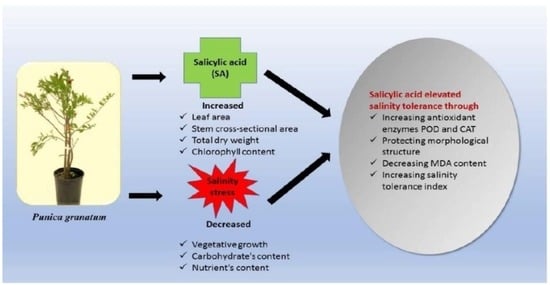
Mitigation of Salinity Stress on Pomegranate (Punica granatum L. cv. Wonderful) Plant Using Salicylic Acid Foliar Spray
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Growth Parameters
2.3. Chlorophyll, Phenol, Carbohydrate, and Proline Content and Peroxidase, and Catalase Activities
2.4. Malondialdehyde (MDA) Content, Electrolyte Leakage (EL), and Mineral Content in Leaves
2.5. Salt Tolerance Index (STI)
2.6. Statistical Analysis
3. Results
3.1. Morphological Performance
3.2. Chlorophyll, Phenol, Carbohydrate, and Proline Content
3.3. Activities of Key Plant Defense-Related Enzymes
3.4. Malondialdehyde (MDA) Content, Electrolyte Leakage (EL), and Mineral Content in Leaves
3.5. Salt Tolerance Index (STI) Percentage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of Rice Rab7 Gene Improves Drought and Heat Tolerance and Increases Grain Yield in Rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Elkelish, A.A.; Alnusaire, T.S.; Soliman, M.H.; Gowayed, S.; Senousy, H.H.; Fahad, S. Calcium availability regulates antioxidant system, physio-biochemical activities and alleviates salinity stress mediated oxidative damage in soybean seedlings. J. Appl. Bot. Food Qual. 2019, 92, 258–266. [Google Scholar]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Bhuyan, M.; Anee, T.I.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In Plant Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2019; pp. 221–257. [Google Scholar]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Samy, M.; Shaimaa, F.; Hoda, A.K. Effect of salicylic acid on growth and physiological status of salt stressed sour orange seedlings (Citrus aurantium L.). Alex. J. Agric. Sci. 2015, 60, 229–239. [Google Scholar] [CrossRef]
- Khalil, H.; El-Ansary, D. Morphological, physiological and anatomical responses of two olive cultivars to deficit irrigation and mycorrhizal inoculation. Eur. J. Hortic. Sci. 2020, 85, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Khalil, H.A. Influence of vesicular-arbuscula mycorrhizal fungi (Glomus spp.) on the response of grapevines rootstocks to salt stress. Asian J. Crop Sci. 2013, 5, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Khalil, H.A.; Eissa, A.M.; El-Shazly, S.M.; Nasr, A.M.A. Improved growth of salinity-stressed citrus after inoculation with mycorrhizal fungi. Sci. Hortic. 2011, 130, 624–632. [Google Scholar] [CrossRef]
- Hara, M.; Furukawa, J.; Sato, A.; Mizoguchi, T.; Miura, K. Abiotic Stress and Role of Salicylic Acid in Plants. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 235–251. [Google Scholar]
- Per, T.S.; Fatma, M.; Asgher, M.; Javied, S.; Khan, N.A. Salicylic Acid and Nutrients Interplay in Abiotic Stress Tolerance. In Salicylic Acid: A Multifaceted Hormone; Nazar, R., Iqbal, N., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 221–237. [Google Scholar]
- Hayat, S.; Ahmad, A. Salicylic Acid-A Plant Hormone; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Hussein, M.; Rezk, A.; AB, E.-N.; HM, M. Nutritional and growth response of canola plants to salicylic acid under salt stress conditions. Int. J. ChemTech Res. 2015, 8, 574–581. [Google Scholar]
- Stevens, J.; Senaratna, T.; Sivasithamparam, K. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul. 2006, 49, 77–83. [Google Scholar]
- Kováčik, J.; Grúz, J.; Bačkor, M.; Strnad, M.; Repčák, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Du, X.; Tang, H.; Shen, C.; Wu, J. Salicylic acid alleviates the adverse effects of salt stress in Torreya grandis cv. Merrillii seedlings by activating photosynthesis and enhancing antioxidant systems. PLoS ONE 2014, 9, e109492. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Bhantana, P.; Lazarovitch, N. Evapotranspiration, crop coefficient and growth of two young pomegranate (Punica granatum L.) varieties under salt stress. Agric. Water Manag. 2010, 97, 715–722. [Google Scholar] [CrossRef]
- Li, J.-G.; Pu, L.-J.; Zhu, M.; Zhang, R. The present situation and hot issues in the salt-affected soil research. Acta Geogr. Sin. 2012, 67, 1233–1245. [Google Scholar]
- Tavousi, M.; Kaveh, F.; Alizadeh, A.; Babazadeh, H.; Tehranifar, A. Effects of drought and salinity on yield and water use efficiency in pomegranate tree. J. Mater. Environ. Sc.i 2015, 6, 1975–1980. [Google Scholar]
- Naeini, M.; Khoshgoftarmanesh, A.; Fallahi, E. Partitioning of chlorine, sodium, and potassium and shoot growth of three pomegranate cultivars under different levels of salinity. J. Plant Nutr. 2006, 29, 1835–1843. [Google Scholar] [CrossRef]
- El-Khawaga, A.S.; Zaeneldeen, E.M.A.; Yossef, M.A. Response of Three Pomegranate Cultivars (Punica granatum L.) to Salinity Stress. Middle East J. Agric. Res. 2013, 1, 64–75. [Google Scholar]
- Sun, Y.; Niu, G.; Masabni, J.G.; Ganjegunte, G. Relative salt tolerance of 22 pomegranate (Punica granatum) cultivars. HortScience 2018, 53, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- Borochov-Neori, H.; Judeinstein, S.; Tripler, E.; Holland, D.; Lazarovitch, N. Salinity effects on colour and health traits in the pomegranate (Punica granatum L.) fruit peel. Int. J. Postharvest Technol. Innov. 2014, 4, 54–68. [Google Scholar] [CrossRef]
- Khalil, H.A.; Eldin, R.M.B. Chitosan improves morphological and physiological attributes of grapevines under deficit irrigation conditions. J. Hortic. Res. 2021, 29, 9–22. [Google Scholar] [CrossRef]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N, N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ahmed, Z.F.R.; Alnuaimi, A.K.H.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste vs. Inorganic Nutrient Solution. Horticulturae 2021, 7, 292. [Google Scholar] [CrossRef]
- Thimmaiah, S.K.T.S.K. Standard Methods of Biochemical Analysis; Kalyani Pub.: New Delhi, India, 1999. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-M.; Lin, C.C.; Kao, C.H. Copper toxicity in rice seedlings: Changes in antioxidative enzyme activities, H2O2 level, and cell wall peroxidase activity in roots. Bot. Bull. Acad. Sin. 2000, 41, 90–103. [Google Scholar]
- Guidi, L.; Tonini, M.; Soldatini, G.F. Effects of high light and ozone fumigation on photosynthesis in Phaseolus vulgaris. Plant Physiol. Biochem. 2000, 38, 717–725. [Google Scholar] [CrossRef]
- Ahmed, Z.F.; Palta, J.P. Lysophosphatidylethanolamine, a natural phospholipid, may retard senescence and improve the shelf life of banana fruit. HortScience 2010, 45, S66. [Google Scholar]
- Khalil, H.A.; Abdelkader, M.F.; Lo’ay, A.; El-Ansary, D.O.; Shaaban, F.K.; Osman, S.O.; Shenawy, I.E.; Osman, H.-E.H.; Limam, S.A.; Abdein, M.A. The Combined Effect of Hot Water Treatment and Chitosan Coating on Mango (Mangifera indica L. cv. Kent) Fruits to Control Postharvest Deterioration and Increase Fruit Quality. Coatings 2022, 12, 83. [Google Scholar] [CrossRef]
- Evenhuis, B.; de Waard, P. Paper 15 Principles and Practices in Plant Analysis. Soil Plant Test. Anal. 1980, 38, 152. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Jackson, R.K.; Brown, J.G. Ante on the Potentionmetric Determination of Chloride; HortScience: Washington, DC, USA, 1955; pp. 65–178. [Google Scholar]
- Sbei, H.; Shehzad, T.; Harrabi, M.; Okuno, K. Salinity tolerance evaluation of Asian barley accessions (Hordeum vulgare L.) at the early vegetative stage. J. Arid Land Studies 2014, 24, 183–186. [Google Scholar]
- Mastrogiannidou, E.; Chatzissavvidis, C.; Antonopoulou, C.; Tsabardoukas, V.; Giannakoula, A.; Therios, I. Response of pomegranate cv. wonderful plants tο salinity. J. Soil Sci. Plant Nutr. 2016, 16, 621–636. [Google Scholar]
- Khayyat, M.; Tehranifar, A.; Davarynejad, G.H.; Sayyari-Zahan, M. Vegetative growth, compatible solute accumulation, ion partitioning and chlorophyll fluorescence of ‘Malas-e-Saveh’and ‘Shishe-Kab’pomegranates in response to salinity stress. Photosynthetica 2014, 52, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhao, X.; Yan, J.; Yuan, Z.; Gu, M. Effects of salt stress on growth, photosynthesis, and mineral nutrients of 18 pomegranate (Punica granatum) cultivars. Agronomy 2020, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Roussos, P.A.; Gasparatos, D.; Kyriakou, C.; Tsichli, K.; Tsantili, E.; Haidouti, C. Growth, nutrient status, and biochemical changes of sour orange plants subjected to sodium chloride stress. Commun. Soil Sci. Plant Anal. 2013, 44, 805–816. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Läuchli, A.; Grattan, S. Plant growth and development under salinity stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–32. [Google Scholar]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef]
- Yildirim, E.; Turan, M.; Guvenc, I. Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. J. Plant Nutr. 2008, 31, 593–612. [Google Scholar] [CrossRef]
- Youssef, R.A.; El-Azab, M.E.; Mahdy, H.A.; Essa, E.M.; Mohammed, K. Effect of salicylic acid on growth, yield, nutritional status and physiological properties of sunflower plant under salinity stress. Int. J. Pharm. Phytopharm. Res. 2017, 7, 54–58. [Google Scholar]
- El-Tayeb, M. Response of barley grains to the interactive e. ect of salinity and salicylic acid. Plant Growth Regul. 2005, 45, 215–224. [Google Scholar] [CrossRef]
- Khokon, M.A.R.; Okuma, E.; Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011, 34, 434–443. [Google Scholar] [CrossRef]
- Khodary, S. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Bagherifard, A.; Bagheri, A.; Sabourifard, H.; Bagherifard, G.; Najar, M. The effect of salicylic acid on some morphological and biochemistry parameters under salt stress in herb artichoke (Cynara scolymus L.). Res. J. Fish. Hydrobiol. 2015, 10, 745–750. [Google Scholar]
- Yin, Y.-G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv.‘Micro-Tom’) fruits in an ABA-and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.-J.; Li, S.-H. Thermotolerance and related antioxidant enzyme activities induced by heat acclimation and salicylic acid in grape (Vitis vinifera L.) leaves. Plant Growth Regul. 2006, 48, 137–144. [Google Scholar] [CrossRef]
- Szalai, G.; Páldi, E.; Janda, T. Effect of salt stress on the endogenous salicylic acid content in maize (Zea mays L.) plants. Acta Biol. Szeged. 2005, 49, 47–48. [Google Scholar]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Zhu, Z. Exogenous salicylic acid alleviates NaCl toxicity and increases antioxidative enzyme activity in Lycopersicon esculentum. Biol. Plant. 2008, 52, 792. [Google Scholar] [CrossRef]
- Shen, C.; Hu, Y.; Du, X.; Li, T.; Tang, H.; Wu, J. Salicylic acid induces physiological and biochemical changes in Torreya grandis cv. Merrillii seedlings under drought stress. Trees 2014, 28, 961–970. [Google Scholar] [CrossRef]
- Ahmed, Z.F.R.; Alblooshi, S.S.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Synergistic effect of preharvest spray application of natural elicitors on storage life and bioactive compounds of date palm (Phoenix dactylifera L., cv. Khesab). Horticulturae 2021, 7, 145. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Grattan, S.; Grieve, C. Salinity–mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Silva, E.; Silveira, J.; Rodrigues, C.; Viégas, R. Physiological adjustment to salt stress in J atropha curcas is associated with accumulation of salt ions, transport and selectivity of K+, osmotic adjustment and K+/N a+ homeostasis. Plant Biol. 2015, 17, 1023–1029. [Google Scholar] [CrossRef]
- Ibrahim, H.I. Tolerance of two pomegranates cultivars (Punica granatum L.) to salinity stress under hydroponic culture conditions. J. Basic. Appl. Sci. Res 2016, 6, 38–46. [Google Scholar]
- Maan, A.A.; Ahmed, Z.F.R.; Khan, M.K.I.; Riaz, A.; Nazir, A. Aloe vera gel, an excellent base material for edible films and coatings. Trends Food Sci. Technol. 2021, 116, 329–341. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Lu, J.; Li, L. Salicylic acid involved in the regulation of nutrient elements uptake and oxidative stress in Vallisneria natans (Lour.) Hara under Pb stress. Chemosphere 2011, 84, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.F.R.; Al Shaibani, F.Y.Y.; Kaur, N.; Maqsood, S.; Schmeda-Hirschmann, G. Improving Fruit Quality, Bioactive Compounds, and Storage Life of Date Palm (Phoenix dactylifera L., cv. Barhi) Using Natural Elicitors. Horticulturae 2021, 7, 293. [Google Scholar] [CrossRef]
- Syeed, S.; Anjum, N.A.; Nazar, R.; Iqbal, N.; Masood, A.; Khan, N.A. Salicylic acid-mediated changes in photosynthesis, nutrients content and antioxidant metabolism in two mustard (Brassica juncea L.) cultivars differing in salt tolerance. Acta Physiol. Plant. 2011, 33, 877–886. [Google Scholar] [CrossRef]
- Tufail, A.; Arfan, M.; Gurmani, A.R.; Khan, A.; Bano, A. Salicylic acid induced salinity tolerance in maize (Zea mays). Pak. J. Bot 2013, 45, 75–82. [Google Scholar]



| 2019 | SCSA | LA | TDW | Chl | LTC | LPC | TPC | EL | CAT | POD | Na | Cl | N | P | Ca | Mg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salinity (S) | 0.25 *** | 665.6 ** | 18.34 ** | 9012.7 ** | 15.93 ** | 144.18 ** | 9.95 *** | 2305.48 * | 9.03 *** | 0.43 *** | 0.16 *** | 0.19 | 0.68 * | 0.04 * | 0.03 * | 0.01 *** |
| SA | 0.04 *** | 4066.0 * | 2.95 *** | 2886.03 * | 18.74 ** | 30.83 *** | 17.64 ** | 532.38 ** | 2.97 *** | 14.96 ** | 0.09 *** | 0.28 * | 0.07 * | 0.01 * | 0.007 * | 0.002 ** |
| S X SA | 0.004 ** | 20.70 * | 0.77 *** | 715.43 ** | 2.14 * | 3.83 * | 0.45NS | 160.12 ** | 0.05 NS | 0.18 ** | 0.01 *** | 0.02 * | 0.06 * | 6.58 * | 4.91 * | 2.41 *** |
| 2020 | SCSA | LA | TDW | Chl | LTC | LPC | TPC | EL | CAT | POD | Na | Cl | N | P | Ca | Mg |
| Salinity (S) | 0.37 *** | 315.2 ** | 1404.9 * | 5457.07 * | 4.42 *** | 38.78 *** | 9.78 *** | 1443.92 * | 17.37 ** | 0.69 *** | 0.23 *** | 0.31 * | 0.92 * | 0.11 * | 0.05 ** | 0.07 *** |
| SA | 0.28 *** | 1078.2 * | 105.69 * | 7383.19 * | 50.76 ** | 61.38 *** | 16.54 ** | 256.22 ** | 5.07 *** | 16.15 ** | 0.05 *** | 0.09 * | 0.37 * | 0.01 * | 0.009 * | 0.07 *** |
| S X SA | 0.02 NS | 7.79 * | 7.47 *** | 708.88 ** | 0.64 *** | 2.58 *** | 0.68 *** | 59.63 *** | 0.1 NS | 0.1 NS | 0.002 ** | 0.01 * | 0.14 * | 4.82 * | 7.91 ** | 0.02 *** |
| Salinity Levels (mM) NaCl | SA Levels (mM) | SCSA (Cm2) | Leaf Area (Cm2) | TDW (g) | |||
|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| 10 | 0 | 0.84 ± 0.2 de | 0.86 ± 1.1 de | 25.8 ± 0.3 g | 32.1 ± 0.2 fg | 37.98 ± 0.5 b | 41.60 ± 0.1 d |
| 0.25 | 0.92 ±0.3 c | 1.15 ± 0.2 bc | 43.5 ± 0.1 e | 45.6 ± 0.1 d | 38.25 ±0.2 a | 43.80 ± 0.7 c | |
| 0.50 | 0.98 ± 0.5 b | 1.26 ± 0.7 b | 56.7 ± 0.2 c | 50.8 ± 0.3 c | 38.32 ± 0.1 a | 48.60 ±0.5 b | |
| 1 | 1.10 ± 1.0 a | 1.50 ± 0.3 a | 78.4 ± 1.1 a | 59.3 ± 0.9 a | 38.34 ± 1.1 a | 52.90 ± 0.5 a | |
| 35 | 0 | 0.75 ± 0.5 f | 0.84 ± 1.2 de | 21.2 ± 0.7 g | 30.1 ± 0.4 g | 35.87 ± 0.9 e | 30.10 ± 0.1 g |
| 0.25 | 0.82 ± 0.7 e | 0.92 ± 0.5 cd | 29.8 ± 0.2 f | 41.2 ± 0.2 e | 36.90 ± 0.3 d | 32.60 ± 0.3 f | |
| 0.50 | 0.88 ± 0.2 cd | 0.95 ± 0.3 cd | 50.1 ± 0.5 d | 46.3 ± 0.2 d | 36.95 ± 0.3 cd | 36.10 ± 0.8 e | |
| 1 | 0.89 ± 0.8 cd | 1.20 ± 0.3 b | 70.2 ± 0.1 b | 53.3 ± 0.8 b | 37.15 ± 0.5 c | 35.90 ± 0.5 e | |
| 70 | 0 | 0.62 ± 0.3 h | 0.70 ± 0.5 e | 15.8 ± 1.1 h | 24.1 ± 0.7 h | 34.75 ± 0.3 e | 22.10 ± 0.7 k |
| 0.25 | 0.66 ± 0.2 gh | 0.79 ± 0.2 e | 25.1 ± 0.3 g | 32.9 ±0.2 f | 34.91 ± 0.2 e | 24.30 ± 0.3 j | |
| 0.50 | 0.69 ± 0.5 g | 0.89 ± 1.1 d | 43.3 ± 0.1 e | 42.6 ± 0.2 e | 36.59 ± 0.1 cd | 27.00 ± 0.3 i | |
| 1 | 0.71 ± 0.8 fg | 0.98 ± 0.1 c | 60.1± 0.5 c | 50.2 ± 0.3 c | 36.73 ± 0.5 cd | 27.60 ± 0.5 h | |
| Salinity Levels (mM) NaCl | SA Levels (mM) | Total Chlorophyll Content (mg−1 100 g DW) | Total Phenolic Composition (% of DW) | Total Carbohydrate Contents (% of DW) | Leaf Proline Content (mg−1 100 g DW) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| 10 | 0 | 177.2 ± 0.03 g | 155.6 ± 0.5 i | 5.12 ± 0.01 f | 5.42 ± 0.01 h | 10.92 ± 0.2 c | 10.88 ± 0.6 e | 12.71 ± 0.6 f | 15.31 ± 0.3 h |
| 0.25 | 200.1 ± 0.05 c | 206.3 ± 0.3 d | 7.11 ± 0.00 de | 6.60 ± 0.01 g | 10.98 ± 0.5 c | 10.90 ± 0.5 e | 12.75 ± 0.1 f | 15.35 ±0.5 h | |
| 0.50 | 252.6 ± 0.04 a | 228.2 ± 0.1 b | 7.92 ± 0.01 cde | 7.81 ±0.01 de | 14.80 ± 0.5 a | 15.13 ± 0.5 b | 12.74 ± 0.5 f | 16.81 ± 0.5 f | |
| 1 | 242.8 ± 0.05 b | 235.1 ± 0.2 a | 8.11 ± 0.01 cd | 7.95 ± 0.02 d | 14.86 ± 0.3 a | 15.60 ± 0.3 a | 17.81 ± 0.3 de | 19.23 ± 0.2 c | |
| 35 | 0 | 173.1 ± 0.02 h | 141.6 ± 0.5 j | 6.80 ± 0.02 e | 6.54 ± 0.00 g | 10.60 ± 0.1 cd | 9.70 ± 0.1 f | 16.11 ± 0.2 e | 16.11 ± 0.1 g |
| 0.25 | 184.2 ± 0.05 f | 178.9 ± 0.5 f | 8.20 ± 0.01 cd | 6.80 ± 0.02 fg | 10.88 ± 0.3 c | 10.89 ± 0.3 e | 17.24 ± 0.4 de | 17.93 ± 0.2 e | |
| 0.50 | 195.6 ± 0.02 d | 206.1 ± 0.4 d | 9.71 ± 0.00 ab | 8.11 ± 0.02 d | 12.70 ± 0.5 b | 13.50 ± 0.3 d | 19.81 ± 0.3 bc | 18.16 ± 0.4 e | |
| 1 | 190.1 ± 0.03 e | 213.1 ± 0.5 c | 9.92 ± 0.02 ab | 8.96 ± 0.02 c | 12.81 ± 0.2 b | 14.90 ± 0.4 b | 20.50 ± 0.5 bc | 22.51 ± 0.2 b | |
| 70 | 0 | 151.7 ± 0.01 j | 131.2 ± 0.3 k | 6.98 ± 0.01 de | 6.92 ± 0.02 f | 9.20 ± 0.1 d | 9.35 ± 0.3 g | 19.11 ± 0.4 cd | 18.15 ± 0.5 e |
| 0.25 | 161.8 ± 0.03 i | 164.1 ± 0.5 h | 8.10 ± 0.01 cd | 7.60 ± 0.02 e | 10.50 ± 0.5 cd | 10.86 ± 0.6 e | 20.95 ± 0.1 abc | 18.50 ± 0.3 d | |
| 0.50 | 170.3 ± 0.05 h | 168.9 ± 0.4 g | 8.93 ± 0.02 bc | 9.50 ± 0.01 b | 11.98 ± 0.3 bc | 13.68 ± 0.3 cd | 21.23 ± 0.3 ab | 19.10 ± 0.5 c | |
| 1 | 170.9 ± 0.04 h | 190.1 ± 0.1 e | 10.5 ± 0.02 a | 10.90 ± 0.01 a | 10.66 ± 0.3 cd | 13.96 ± 0.5 c | 22.60 ± 0.3 a | 25.30 ± 0.3 a | |
| Salinity Levels (mM) | SA Levels (mM) | Na (% DW) | Cl (% DW) | EL (%) | |||
|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| 10 | 0 | 0.52 ± 0.01 ef | 0.61 ± 0.02 f | 0.71 ± 0.05 de | 0.85 ± 0.05 cd | 20.1 ± 0.1 h | 18.2 ± 0.5 h |
| 0.25 | 0.50 ± 0.05 fg | 0.59 ± 0.01 f | 0.70 ± 0.04 ef | 0.80 ± 0.03 d | 19.2 ± 0.3 hi | 15.1 ± 0.2 i | |
| 0.50 | 0.45 ± 0.03 h | 0.47 ± 0.04 g | 0.53 ± 0.03 gh | 0.60 ± 0.03 e | 15.5 ± 0.4 j | 15.3 ± 0.4 i | |
| 1 | 0.46 ± 0.01 h | 0.42 ± 0.05 lh | 0.50 ± 0.03 g | 0.56 ± 0.01 e | 18.5 ± 0.3 i | 18.4 ± 0.1 h | |
| 35 | 0 | 0.70 ± 0.05 c | 0.79 ± 0.06 bc | 0.92 ± 0.05 b | 1.02 ± 0.05 b | 40.5 ± 0.5 b | 39.6 ± 0.1 c |
| 0.25 | 0.69 ± 0.01 c | 0.71 ± 0.03 d | 0.86 ± 0.02 bc | 0.90 ± 0.02 f | 30.2 ± 0.2 e | 29.1 ± 0.3 f | |
| 0.50 | 0.50 ± 0.02 fg | 0.69 ± 0.02 d | 0.69 ± 0.01 ef | 0.90 ± 0.05 bcd | 28.1 ± 0.1 f | 25.2 ± 0.4 g | |
| 1 | 0.48 ± 0.04 gh | 0.65 ± 0.03 e | 0.55 ± 0.05 gh | 0.88 ± 00.6 bcd | 26.1 ± 0.5 g | 25.1± 0.3 g | |
| 70 | 0 | 0.89 ± 0.03 a | 0.92 ± 0.02 a | 1.20 ± 0.03 a | 1.18 ± 0.01 a | 69.1 ± 0.3 a | 50.1 ± 0.2 a |
| 0.25 | 0.82 ± 0.05 b | 0.80 ± 0.01 b | 0.95 ± 0.02 b | 1.0 ± 0.03 bc | 40.2 ± 0.4 b | 40.1 ± 0.5 b | |
| 0.50 | 0.60 ± 0.02 d | 0.77 ± 0.03 c | 0.80 ± 0.05 cd | 0.98 ± 0.02 bc | 38.5 ± 0.4 c | 33.2 ± 0.1 d | |
| 1 | 0.55 ± 0.01 e | 0.70 ± 0.05 d | 0.61 ± 0.01 fg | 0.91 ± 0.05 bcd | 36.3 ± 0.5 d | 30.7 ± 0.3 e | |
| Salinity Levels (mM) NaCl | SA Levels (mM) | N (% DW) | P (% DW) | K (% DW) | Ca (% DW) | Mg (% DW) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| 10 | 0 | 2.4 ± 0.01 c | 2.4 ± 0.03 ab | 0.54 ± 0.05 c | 0.45 ± 0.04 b | 1.8 ± 0.03 a | 1.6 ± 0.03 a | 1.8 ±0.06 a | 1.4 ± 0.01 a | 1.7 ± 0.01 a | 1.7 ± 0.01 a |
| 0.25 | 2.5 ± 0.05 c | 2.4 ± 0.08 a | 0.55 ± 0.03 bc | 0.55 ± 0.03 a | 1.8 ± 0.05 a | 1.6 ± 0.05 a | 1.9 ± 0.03 a | 1.4 ± 0.02 a | 1.7 ± 0.02 a | 1.7 ± 0.02 a | |
| 0.50 | 3.4 ± 0.04 b | 2.4 ± 0.08 a | 0.58 ± 0.03 b | 0.56 ± 0.03 a | 1.8 ± 0.02 a | 1.6 ± 0.05 a | 1.7 ± 0.02 a | 1.4 ± 0.04 a | 1.7 ± 0.03 a | 1.7 ± 0.03 a | |
| 1 | 3.9 ± 0.03 a | 2.4 ± 0.07 a | 0.68 ± 0.02 a | 0.61 ± 0.04 a | 1.8 ± 0.02 a | 1.6 ± 0.05 a | 1.9 ± 0.01 a | 1.4 ± 0.05 a | 1.7 ± 0.05 a | 1.7 ± 0.02 a | |
| 35 | 0 | 2.4 ± 0.06 c | 1.9 ± 0.08 cd | 0.34 ± 0.05 c | 0.37 ± 0.03 c | 1.2 ± 0.02 b | 1.2 ± 0.03 b | 1.3 ± 0.05 c | 1.2 ± 0.02 c | 1.6 ± 0.01 b | 1.5 ± 0.04 b |
| 0.25 | 2.7 ± 0.05 b | 2.1 ± 0.09 c | 0.39 ± 0.03 bc | 0.42 ± 0.04 b | 1.6 ± 0.05 a | 1.6 ± 0.02 a | 1.5 ± 0.01 b | 1.3 ± 0.01 bc | 1.6 ± 0.03 a | 1.6±0.05 ab | |
| 0.50 | 2.9 ± 0.3 b | 2.2 ± 0.08 b | 0.49 ± 0.03 b | 0.41 ± 0.05 b | 1.7 ± 0.02 a | 1.7 ± 0.01 a | 1.6 ± 0.01 b | 1.3 ± 0.02 b | 1.6 ± 0.01 a | 1.6 ± 0.01 a | |
| 1 | 3.7 ± 0.09 a | 2.3 ± 0.07 b | 0.62 ± 0.04 a | 0.44 ± 0.05 b | 1.8 ± 0.03 a | 1.8 ± 0.03 a | 1.7 ± 0.04 b | 1.3 ± 0.01 b | 1.6 ± 0.05 a | 1.7 ± 0.05 a | |
| 70 | 0 | 1.8 ± 0.08 c | 1.8 ± 0.08 d | 0.36 ± 0.05 b | 0.30± 0.05 d | 1.1 ± 0.03 c | 1.1 ± 0.02 c | 1.3 ± 0.01 d | 1.2 ± 0.03 c | 1.5 ± 0.03 b | 1.5 ± 0.05 b |
| 0.25 | 2.1 ± 0.06 bc | 1.8 0.05 d | 0.40 ± 0.02 a | 0.35 ± 0.02 c | 1.3 ± 0.05 b | 1.4 ± 0.01 b | 1.3 ± 0.03 bc | 1.2 ± 0.05 c | 1.6 ± 0.01 a | 1.5± 0.05 ab | |
| 0.50 | 2.4 ± 0.05 ab | 1.9 ± 0.09 c | 0.40 ± 0.02 a | 0.37 ± 0.05 c | 1.3 ± 0.03 b | 1.5 ± 0.02 b | 1.4 ± 0.01 bc | 1.3 ± 0.03 b | 1.6 ± 0.02 ab | 1.6± 0.05 ab | |
| 1 | 2.7 ± 0.05 a | 2.1 ± 0.08 c | 0.40 0.03 a | 0.40 ± 0.03 c | 1.7 ± 0.05 a | 1.6 ± 0.01 a | 1.4 ± 0.05 b | 1.3 ± 0.01 b | 1.6 ± 0.01 a | 1.7 ± 0.05 a | |
| Trait | STI (%) | |
|---|---|---|
| 2019 | 2020 | |
| Stem cross-sectional area (SCSA) | 73.80 ± 0.9 ef | 81.39 ±1.1 de |
| Leaf area | 61.24 ± 1.2 f | 75.07 ± 1.2 ef |
| Total dry weight | 91.49 ± 1.1 e | 92.18 ± 1.1 d |
| Chlorophyll | 85.60 ± 0.5 ef | 84.31 ± 0.9 de |
| Phenolic composition | 136.32 ± 0.3 c | 127.67 ± 0.05 c |
| Carbohydrates | 84.24 ± 0.9 ef | 85.93 ± 0.05 de |
| Proline | 150.35 ± 0.2 c | 118.54 ± 0.01 c |
| Catalase (CAT) | 128.84 ± 0.05 d | 141.17 ± 0.02 b |
| Peroxidase (POD) | 110.25 ± 0.06 d | 115.00 ± 0.01 c |
| Na (%) | 171.15 ± 0.05 b | 150.81 ± 0.02 b |
| Cl (%) | 169.01 ± 0.02 b | 138.82 ± 0.02 b |
| Electrolyte leakage (EL) | 343.78 ± 0.05 a | 275.27 ± 0.08 a |
| N (%) | 75.40 ± 0.08 ef | 74.15 ± 0.05 ef |
| P (%) | 66.66 ± 0.05 f | 66.67 ± 0.02 f |
| K (%) | 59.44 ± 0.03 f | 72.25 ± 0.01 ef |
| Ca (%) | 70.65 ± 0.02 ef | 90.73 ± 0.02 d |
| Mg (%) | 90.47 ± 0.01 e | 88.30 ± 0.01 de |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, H.A.; El-Ansary, D.O.; Ahmed, Z.F.R. Mitigation of Salinity Stress on Pomegranate (Punica granatum L. cv. Wonderful) Plant Using Salicylic Acid Foliar Spray. Horticulturae 2022, 8, 375. https://doi.org/10.3390/horticulturae8050375
Khalil HA, El-Ansary DO, Ahmed ZFR. Mitigation of Salinity Stress on Pomegranate (Punica granatum L. cv. Wonderful) Plant Using Salicylic Acid Foliar Spray. Horticulturae. 2022; 8(5):375. https://doi.org/10.3390/horticulturae8050375
Chicago/Turabian StyleKhalil, Hoda A., Diaa O. El-Ansary, and Zienab F. R. Ahmed. 2022. "Mitigation of Salinity Stress on Pomegranate (Punica granatum L. cv. Wonderful) Plant Using Salicylic Acid Foliar Spray" Horticulturae 8, no. 5: 375. https://doi.org/10.3390/horticulturae8050375
APA StyleKhalil, H. A., El-Ansary, D. O., & Ahmed, Z. F. R. (2022). Mitigation of Salinity Stress on Pomegranate (Punica granatum L. cv. Wonderful) Plant Using Salicylic Acid Foliar Spray. Horticulturae, 8(5), 375. https://doi.org/10.3390/horticulturae8050375