Abrupt Spin Crossover Behavior in a Linear N1,N2-Triazole Bridged Trinuclear Fe(II) Complex
Abstract
:1. Introduction
2. Experimental Section
2.1. General Methods and Materials
2.2. Single Crystal X-ray Diffraction
2.3. Syntheses
2.3.1. Synthesis of the Ligand
2.3.2. Synthesis of the Complex
Synthesis of [FeII2(pyrtrz)5(SCN)4]·7H2O (C1)
Synthesis of [FeII3(pyrtrz)6(TsO)6]·10H2O·2CH3OH (C2)
3. Rerult and Discussion
3.1. X-ray Crystal Structure Description
3.1.1. Crystal Structure of [FeII2(pyrtrz)5(SCN)4]·7H2O (C1)
3.1.2. Crystal Structure of [FeII3(pyrtrz)6(TsO)6]·10H2O·2CH3OH (C2)
3.2. FT-IR Spectroscopy
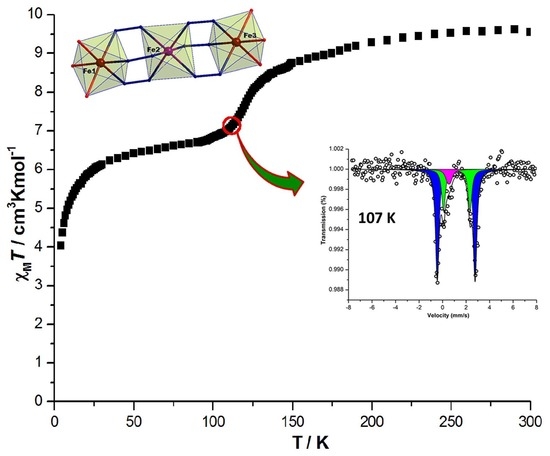
3.3. Magnetic Susceptibility and Mössbauer Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Halcrow, M.A. Iron(II) complexes of 2,6-di(pyrazol-1-yl)pyridines—A versatile system for spin-crossover research. Coord. Chem. Rev. 2009, 253, 2493–2514. [Google Scholar] [CrossRef]
- Berdiell, I.C.; Kulmaczewski, R.; Halcrow, M.A. Iron(II) Complexes of 2,4-Dipyrazolyl-1,3,5-triazine Derivatives—The Influence of Ligand Geometry on Metal Ion Spin State. Inorg. Chem. 2017, 56, 8817–8828. [Google Scholar] [CrossRef] [PubMed]
- Deeth, R.J.; Halcrow, M.A.; Kershaw Cook, L.J.; Raithby, P.R. Ab Initio Ligand Field Molecular Mechanics and the Nature of Metal-Ligand π-Bonding in Fe(II) 2,6-di(pyrazol-1-yl)pyridine Spin Crossover Complexes. Chem. Eur. J. 2018, 24, 5204–5212. [Google Scholar] [CrossRef] [PubMed]
- Kershaw Cook, L.J.; Thorp-Greenwood, F.L.; Comyn, T.P.; Cespedes, O.; Chastanet, G.; Halcrow, M.A. Unexpected Spin-Crossover and a Low-Pressure Phase Change in an Iron(II)/Dipyrazolylpyridine Complex Exhibiting a High-Spin Jahn–Teller Distortion. Inorg. Chem. 2015, 54, 6319–6330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feltham, H.L.; Barltrop, A.S.; Brooker, S. Spin crossover in iron(II) complexes of 3,4,5-tri-substituted-1,2,4-triazole (Rdpt), 3,5-di-substituted-1,2,4-triazolate (dpt−), and related ligands. Coord. Chem. Rev. 2017, 344, 26–53. [Google Scholar] [CrossRef]
- Feltham, H.L.; Dankhoff, K.; Meledandri, C.J.; Brooker, S. Towards Dual-Functionality Spin-Crossover Complexes. Chem. Plus Chem. 2018, 83, 582–589. [Google Scholar] [CrossRef]
- Feltham, H.L.; Cowan, M.G.; Kitchen, J.A.; Jameson, G.N.; Brooker, S. Targeted structural modification of spin crossover complexes: pyridine vs pyrazine. Supramol. Chem. 2018, 30, 296–304. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, S.; Barltrop, A.S.; White, N.G.; Feltham, H.L.; Brooker, S. Solvent Polarity Predictably Tunes Spin Crossover T1/2 in Isomeric Iron(II) Pyrimidine Triazoles. Inorg. Chem. 2018, 57, 6266–6282. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.F.; Carrella, L.M.; Rentschler, E. A Family of Dinuclear Iron(II) SCO Compounds Based on a 1,3,4-Thiadiazole Bridging Ligand. Eur. J. Inorg. Chem. 2015, 22, 3632–3636. [Google Scholar] [CrossRef]
- Köhler, C.; Rentschler, E. The First 1,3,4-Oxadiazole Based Dinuclear Iron(II) Complexes Showing Spin Crossover Behavior with Hysteresis. Eur. J. Inorg. Chem. 2016, 13, 1955–1960. [Google Scholar] [CrossRef]
- Haasnoot, J.G. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivatives as ligands. Coord. Chem. Rev. 2000, 200, 131–185. [Google Scholar] [CrossRef]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef] [Green Version]
- Roubeau, O. Triazole-based one-dimensional spin-crossover coordination polymers. Chem. Eur. J. 2012, 18, 15230–15244. [Google Scholar] [CrossRef] [PubMed]
- Lavrenova, L.G.; Shakirova, O.G. Spin Crossover and Thermochromism of Iron(II) Coordination Compounds with 1, 2, 4-Triazoles and Tris (pyrazol-1-yl) methanes. Eur. J. Inorg. Chem. 2013, 670–682. [Google Scholar] [CrossRef]
- Garcia, Y.; Niel, V.; Muñoz, M.C.; Real, J.A. Spin Crossover in 1D, 2D and 3D Polymeric Fe(II) Networks. In Spin Crossover in Transition Metal Compounds I; Gütlich, P., Goodwin, H.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 233, pp. 229–257. [Google Scholar]
- Kahn, O.; Jay, M.C. Spin-transition polymers: from molecular materials toward memory devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Grosjean, A.; Daro, N.; Kauffmann, B.; Kaiba, A.; Létard, J.F.; Guionneau, P. The 1-D polymeric structure of the [Fe(NH2trz)3](NO3)2·nH2O (with n = 2) spin crossover compound proven by single crystal investigations. Chem. Commun. 2011, 47, 12382–12384. [Google Scholar] [CrossRef] [PubMed]
- Pittala, N.; Thétiot, F.; Triki, S.; Boukheddaden, K.; Chastanet, G.; Marchivie, M. Cooperative 1D Triazole-Based Spin Crossover FeII Material with Exceptional Mechanical Resilience. Chem. Mater. 2016, 29, 490–494. [Google Scholar] [CrossRef]
- Klein, Y.M.; Sciortino, N.F.; Housecroft, C.E.; Kepert, C.J.; Neville, S.M. Structure and Magnetic Properties of the Spin Crossover Linear Trinuclear Complex [Fe3(furtrz)6(ptol)2(MeOH)4]·4(ptol)·4(MeOH) (furtrz: furanylidene-4H-1,2,4-triazol-4-amine ptol: p-tolylsulfonate). Magnetochemistry 2016, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Liu, Q.; Meng, Y.S.; Zheng, H.; Zhu, H.L.; Shi, Q.; Liu, T. Synergic on/off Photoswitching Spin State and Magnetic Coupling between Spin Crossover Centers. Inorg. Chem. 2017, 56, 10674–10680. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Leng, J.D.; Wang, Z.Z.; Chen, Y.C.; Miao, Y.; Tong, M.L.; Dong, W. Reversible crystal-to-crystal transformation from a trinuclear cluster to a 1D chain and the corresponding spin crossover (SCO) behaviour change. Chem. Commun. 2017, 53, 7820–7823. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Chen, Y.C.; Yang, M.; Tong, M.L.; Dong, W. Water molecule induced reversible single-crystal-to-single-crystal transformation between two trinuclear Fe (ii) complexes with different spin crossover behavior. Dalton Trans. 2018, 47, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Pittala, N.; Thétiot, F.; Charles, C.; Triki, S.; Boukheddaden, K.; Chastanet, G.; Marchivie, M. An unprecedented trinuclear Fe II triazole-based complex exhibiting a concerted and complete sharp spin transition above room temperature. Chem. Commun. 2017, 53, 8356–8359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomann, M.; Kahn, O.; Guilhem, J.; Varret, F. Spin conversion versus antiferromagnetic interaction in iron (II) trinuclear species. crystal structures and magnetic properties of [Fe3(p-MeOptrz)8(H2O)4](BF4)6 and [Fe3(p-MeOprtz)6(H2O)6](tos)6 [p-MeOptrz= 4-(p-Methoxyphenyl)-1, 2, 4-triazole, tos= tosylate]. Inorg. Chem. 1994, 33, 6029–6037. [Google Scholar]
- Kolnaar, J.J.A.; van Dijk, G.; Koojiman, H.; Spek, A.L.; Ksenofontov, V.; Gütlich, P.; Haasnoot, J.G.; Reedijk, J. Synthesis, Structure, Magnetic Behavior, and Mössbauer Spectroscopy of Two New Iron (II) Spin-Transition Compounds with the Ligand 4-Isopropyl-1, 2, 4-triazole. X-ray Structure of [Fe3(4-isopropyl-1, 2, 4-triazole)6(H2O)6](tosylate)6·2H2O. Inorg. Chem. 1997, 36, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Savard, D.; Cook, C.; Enright, G.D.; Korobkov, I.; Burchell, T.J.; Murugesu, M. Gradual spin crossover behaviour in a linear trinuclear Fe II complex. Cryst. Eng. Comm. 2011, 13, 5190–5197. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Gunnlaugsson, H.P. Spreadsheet based analysis of Mössbauer spectra. Hyperfine Interact. 2016, 237, 79. [Google Scholar] [CrossRef]
- SMART 5.0 and SAINT 4.0 for Windows NT, Area Detector Control and Integration Software; Bruker Analytical X-Ray Systems Inc.: Madison, WI, USA, 1998.
- Sheldrick, G.M. SADABS: Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Sheldrick, G.M. SHELXTL 5.1 for Windows NT: Structure Determination Software Programs; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Diamond—Crystal and Molecular Structure Visualization. Available online: http://www.crystalimpact.com/diamond (accessed on 5 August 2018).
- Khan, T.; Yadav, R. Synthesis, characterization and antioxidant activity of some new 4-thiazolidinonyl-4H-1, 2, 4-triazole derivatives. Heterocycl. Lett. 2016, 6, 757–766. [Google Scholar]
- Kwiecień, A.; Barys, M.; Ciunik, Z. Stable Hemiaminals with a Cyano Group and a Triazole Ring. Molecules 2014, 19, 11160–11177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, P.; Tissot, A.; Peterhans, L.; Guenee, L.; Besnard, C.; Pattison, P.; Hauser, A. Determination of the molecular structure of the short-lived light-induced high-spin state in the spin-crossover compound [Fe(6-mepy)3tren](PF6)2. Phys. Rev. B 2013, 87, 214306. [Google Scholar] [CrossRef]
- Gómez, V.; de Pipaón, C.S.; Maldonado-Illescas, P.; Waerenborgh, J.C.; Martin, E.; Benet-Buchholz, J.; Galan-Máscarós, J.R. Easy Excited-State Trapping and Record High T TIESST in a Spin-Crossover Polyanionic FeII Trimer. J. Am. Chem. Soc. 2015, 137, 11924–11927. [Google Scholar] [CrossRef] [PubMed]
- Borello, E.; Zecchina, A. Infrared spectra of v-triazoles—I: 2-Aryl-v-triazoles. Spectrochim. Acta 1963, 19, 1703–1715. [Google Scholar] [CrossRef]
- Prashanthi, Y.; Raj, S. Synthesis and characterization of transition metal complexes with N, O; N, N and S, N-donor Schifff base ligands. J. Sci. Res. 2010, 2, 114–126. [Google Scholar]
- Sumrra, S.H.; Chohan, Z.H. Spectrochimica acta A: Molecular and biomolecular spectroscopy. Spectrochim. Acta 2012, 98, 53–61. [Google Scholar]
- Salaudeen, A.A.; Kilner, C.A.; Halcrow, M.A. Mononuclear and dinuclear iron thiocyanate and selenocyanate complexes of tris-pyrazolylmethane ligands. Polyhedron 2008, 12, 2569–2576. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B, 5th ed.; Wiley Interscience: New York, NY, USA, 1997; p. 116. [Google Scholar]
- Arduini, A.L.; Garnett, M.; Thompson, R.C.; Wong, T.C.T. Magnetic and Spectral Studies on Cobalt (II) and Copper (II) Salts of Methylsulfuric, Trifluoromethylsulfuric, and Paratolylsulfuric Acids. Can. J. Chem. 1975, 53, 3812–3819. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, V.; Winkler, H. Structure and dynamics of biomolecules studied by Mössbauer spectroscopy. Rep. Prog. Phys. 2000, 63, 263–353. [Google Scholar] [CrossRef]
- Jung, J.; Spiering, H.; Yu, Z.; Gütlich, P. The debye-waller factor in spin crossover molecular crystals: A mössbauer study on [FexZn1−x(ptz)6](BF4)2. Hyperfine Interact. 1995, 95, 107–128. [Google Scholar] [CrossRef]
- Kolnaar, A.; Jeroen, J.; de Heer, M.I.; Kooijman, H.; Spek, A.L.; Schmitt, G.; Ksenofontov, V.; Gütlich, P.; Haasnoot, J.G.; Reedijk, J. Synthesis, Structure and Properties of a Mixed Mononuclear/Dinuclear Iron (II) Spin-Crossover Compound with the Ligand 4-(p-Tolyl)-1, 2,4-triazole. Eur. J. Inorg. Chem. 1999, 5, 881–886. [Google Scholar] [CrossRef]











| Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C39H49Fe2N29O7S4 | C86H116Fe3N30O32S6 |
| Formula weight | 1213.90 | 2393.76 |
| Crystal size | 0.14 × 0.13 × 0.07 | 0.51 × 0.11 × 0.03 |
| Crystal system | Triclinic | Triclinic |
| Space group | P-1 (No. 2) | P121/c1 (No. 14) |
| a (Å) | 11.9168(13) | 19.3399(19) |
| b (Å) | 13.8948(15) | 15.6171(15) |
| c (Å) | 18.830(2) | 37.287(4) |
| α (°) | 90.543(3) | 90 |
| β (°) | 101.891(3) | 96.902(2) |
| γ (°) | 102.381(3) | 90 |
| V (Å3) | 2975.4(6) | 1,1180.2(19) |
| Z | 2 | 4 |
| Dcalc (g/cm3) | 1.355 | 1.422 |
| μ(Mo-Kα) (mm−1) | 0.691 | 0.583 |
| F(000) | 1240 | 4936 |
| Reflections collected | 28,747 | 64,247 |
| Independent reflections | 10,485 (0.1442) | 26,617 (0.1035) |
| Parameters | 668 | 1858 |
| Goodness-of-fit | 0.919 | 0.996 |
| R1 [I > 2σ(I)] a | 0.1123 | 0.0852 |
| wR2 (all data) b | 0.3641 | 0.2743 |
| T = 77 K | |||
| Subspectra | HS Fe1 and Fe3 | LS Fe2 | HS Fe2 and Fe3 |
| δ [mm/s] | 1.22 ± 0.02 | 0.54 ± 0.04 | - |
| ΔE0 [mm/s] | 3.22 ± 0.02 | 0.24 ± 0.03 | - |
| Γ [mm/s] | 0.41 ± 0.03 | 0.47 ± 0.03 | - |
| Rel. Area [%] | 66.00 ± 1.00 | 34.00 ± 1.00 | 0 |
| T = 107 K | |||
| δ [mm/s] | 1.16 ± 0.03 | 0.54 ± 0.05 | 1.21 ± 0.04 |
| ΔE0 [mm/s] | 3.22 ± 0.04 | 0.24 ± 0.10 | 2.25 ± 0.02 |
| Γ [mm/s] | 0.34 ± 0.02 | 0.47 ± 0.07 | 0.36 ± 0.04 |
| Rel. Area [%] | 65.00 ± 1.00 | 8.00 ± 2.00 | 27.00 ± 1.00 |
| T = 137 K | |||
| δ [mm/s] | 1.16 ± 0.03 | - | 1.21 ± 0.03 |
| ΔE0 [mm/s] | 3.22 ± 0.04 | - | 2.05 ± 0.04 |
| Γ [mm/s] | 0.34 ± 0.02 | - | 0.44 ± 0.03 |
| Rel. Area [%] | 65.00 ± 2.00 | - | 35.00 ± 1.00 |
| T = 167 K | |||
| δ [mm/s] | 1.15 ± 0.02 | - | 1.21 ± 0.04 |
| ΔE0 [mm/s] | 3.16 ± 0.02 | - | 1.93 ± 0.03 |
| Γ [mm/s] | 0.34 ± 0.01 | - | 0.36 ± 0.05 |
| Rel. Area [%] | 64.00 ± 2.00 | - | 36.00 ± 2.00 |
| T = 197 K | |||
| δ [mm/s] | 1.15 ± 0.04 | - | 1.21 ± 0.03 |
| ΔE0 [mm/s] | 3.16 ± 0.04 | - | 1.87 ± 0.04 |
| Γ [mm/s] | 0.34 ± 0.02 | - | 0.36 ± 0.01 |
| Rel. Area [%] | 67.00 ± 1.00 | - | 33.00 ± 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.-M.; Hochdörffer, T.; Wolny, J.A.; Schünemann, V.; Rentschler, E. Abrupt Spin Crossover Behavior in a Linear N1,N2-Triazole Bridged Trinuclear Fe(II) Complex. Magnetochemistry 2018, 4, 34. https://doi.org/10.3390/magnetochemistry4030034
Li A-M, Hochdörffer T, Wolny JA, Schünemann V, Rentschler E. Abrupt Spin Crossover Behavior in a Linear N1,N2-Triazole Bridged Trinuclear Fe(II) Complex. Magnetochemistry. 2018; 4(3):34. https://doi.org/10.3390/magnetochemistry4030034
Chicago/Turabian StyleLi, Ai-Min, Tim Hochdörffer, Juliusz A. Wolny, Volker Schünemann, and Eva Rentschler. 2018. "Abrupt Spin Crossover Behavior in a Linear N1,N2-Triazole Bridged Trinuclear Fe(II) Complex" Magnetochemistry 4, no. 3: 34. https://doi.org/10.3390/magnetochemistry4030034
APA StyleLi, A. -M., Hochdörffer, T., Wolny, J. A., Schünemann, V., & Rentschler, E. (2018). Abrupt Spin Crossover Behavior in a Linear N1,N2-Triazole Bridged Trinuclear Fe(II) Complex. Magnetochemistry, 4(3), 34. https://doi.org/10.3390/magnetochemistry4030034