Magnetostructural Studies on Zigzag One-Dimensional Coordination Polymers Formed by Tetraamidatodiruthenium(II,III) Paddlewheel Units Bridged by SCN Ligands
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterization
2.2. Crystal Structures
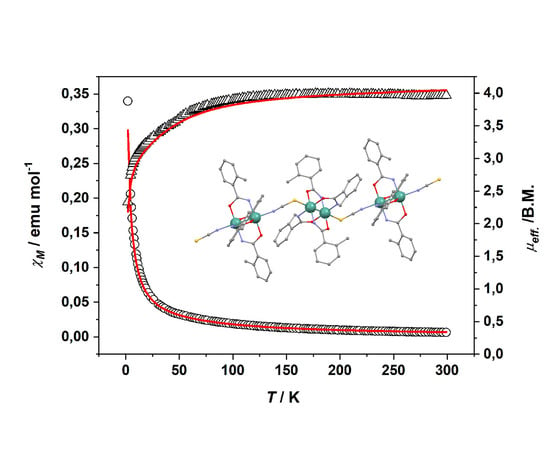
2.3. Magnetic Properties
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Synthesis
3.2.1. Synthesis of [Ru2(μ-NHOCC6H4-o-Me)4(THF)2](BF4) (1)
3.2.2. Synthesis of [Ru2(SCN)(μ-NHOCR)4]n (R = o-Me-C6H4 (2), m-Me-C6H4 (3), p-Me-C6H4 (4))
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cotton, F.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 2nd ed.; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 3rd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Aquino, M.A.S. Diruthenium and diosmium tetracarboxylates: Synthesis, physical properties and applications. Coord. Chem. Rev. 1998, 170, 141–202. [Google Scholar] [CrossRef]
- Aquino, M.A.S. Recent developments in the synthesis and properties of diruthenium tetracarboxylates. Coord. Chem. Rev. 2004, 248, 1025–1045. [Google Scholar] [CrossRef]
- Liddle, S.T. (Ed.) Molecular Metal-Metal Bonds: Compounds, Synthesis, Properties; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Malinski, T.; Chang, D.; Feldmann, F.N.; Bear, J.L.; Kadish, K.M. Electrochemical Studies of a Novel Ruthenium(II, III) Dimer, Ru2(HNOCCF3)4Cl. Inorg. Chem. 1983, 22, 3225–3233. [Google Scholar] [CrossRef]
- Chavan, M.Y.; Feldmann, F.N.; Lin, X.Q.; Bear, J.L.; Kadish, K.M. Electrochemical Generation of New Dinuclear Ruthenium Acetamidate Complexes. Inorg. Chem. 1984, 23, 2373–2375. [Google Scholar] [CrossRef]
- Barral, M.C.; Jiménez-Aparicio, R.; Monge, A.; Priego, J.L.; Royer, E.C.; Ruíz-Valero, C.; Urbanos, F.A. Tert-Butylbenzamidate diruthenium(II, III) Compounds. Crystal Structure of [Ru2(μ-HNOC6H4-p-CMe3)4(OPPh3)2[BF4]. Polyhedron 1993, 12, 2947–2953. [Google Scholar] [CrossRef]
- Chakravarty, A.R.; Cotton, F.A.; Tocher, D.A. Synthesis and Structure of a Binuclear Ruthenium 4-Chloro-Benzamidato Complex. Polyhedron 1985, 4, 1097–1102. [Google Scholar] [CrossRef]
- Chakravarty, A.R.; Cotton, F.A. Structure of a Diruthenium(II, III) Complex with Benzamidato Bridging Ligands. Polyhedron 1985, 4, 1957–1958. [Google Scholar] [CrossRef]
- Ryde, K.; Tocher, D.A. The Electro-oxidation of the Binuclear Ruthenium(II/III) Tetra-amidate Complex, Ru2(Me3CCONH)4Cl. Inorg. Chim. Acta 1986, 118, L49–L51. [Google Scholar] [CrossRef]
- Delgado, P.; González-Prieto, R.; Jiménez-Aparicio, R.; Perles, J.; Priego, J.L.; Torres, M.R. Comparative study of different methods for the preparation of tetraamidato and tetracarboxylatodiruthenium compounds. Structural and magnetic characterization. Dalton Trans. 2012, 41, 11866–11874. [Google Scholar] [CrossRef]
- Delgado-Martínez, P.; González-Prieto, R.; Gómez-García, C.J.; Jiménez-Aparicio, R.; Priego, J.L.; Torres, M.R. Structural, magnetic and electrical properties of one-dimensional tetraamidatodiruthenium compounds. Dalton Trans. 2014, 43, 3227–3237. [Google Scholar] [CrossRef]
- Delgado-Martínez, P.; Freire, C.; González-Prieto, R.; Jiménez-Aparicio, R.; Priego, J.L.; Torres, M.R. Synthesis, Crystal Structure, and Magnetic Properties of Amidate and Carboxylate Dimers of Ruthenium. Crystals 2017, 7, 192. [Google Scholar] [CrossRef]
- Villalobos, L.; Cao, Z.; Fanwick, P.E.; Ren, T. Diruthenium(II, III) tetramidates as a new class of oxygenation catalysts. Dalton Trans. 2012, 41, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, M.; Fuma, Y. Two- and three-dimensional assembled structures constructed from amidate-bridged paddlewheel complexes with group 6 oxometallate ions. Acta Cryst. 2013, B69, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J. Ambidentate ligands, the schizophrenics of coordination chemistry. Coord. Chem. Rev. 1990, 105, 77–133. [Google Scholar] [CrossRef]
- Brewster, T.P.; Ding, W.; Schley, N.D.; Hazari, N.; Batista, V.S.; Crabtree, R.H. Thiocyanate Linkage Isomerism in a Ruthenium Polypyridyl Complex. Inorg. Chem. 2011, 50, 11938–11946. [Google Scholar] [CrossRef]
- Mautner, F.A.; Albering, J.H.; Harrelson, E.V.; Gallo, A.A.; Massoud, S.S. N-bonding vs. S-bonding in thiocyanato-copper(II) complexes. J. Mol. Struct. 2011, 1006, 570–575. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Rashmawi, L.G.; Louka, F.R.; Massoud, S.S. Structural characterization of metal(II) thiocyanato complexes derived from bis(2-(H-pyrazol-1-yl)ethyl)amine. Polyhedron 2017, 124, 237–242. [Google Scholar] [CrossRef]
- Mekuimemba, C.D.; Conan, F.; Mota, A.J.; Palacios, M.A.; Colacio, E.; Triki, S. On the Magnetic Coupling and Spin Crossover Behavior in Complexes Containing the Head-to-Tail [Fe2II(μ-SCN)2] Bridging Unit: A Magnetostructural Experimental and Theoretical Study. Inorg. Chem. 2018, 57, 2184–2192. [Google Scholar] [CrossRef]
- Rams, M.; Tomkowicz, Z.; Böhme, M.; Plass, W.; Suckert, S.; Werner, J.; Jessc, I.; Näther, C. Influence of metal coordination and co-ligands on the magnetic properties of 1D Co(NCS)2 coordination polymers. Phys. Chem. Chem. Phys. 2017, 19, 3232–3243. [Google Scholar] [CrossRef]
- Shurdha, E.; Moore, C.E.; Rheingold, A.L.; Lapidus, S.H.; Stephens, P.W.; Arif, A.M.; Miller, J.S. First Row Transition Metal(II) Thiocyanate Complexes, and Formation of 1-, 2-, and 3-Dimensional Extended Network Structures of M(NCS)2(Solvent)2 (M = Cr, Mn, Co) Composition. Inorg. Chem. 2013, 52, 10583–10594. [Google Scholar] [CrossRef]
- Nebbali, K.; Mekuimemba, C.D.; Charles, C.; Yefsah, S.; Chastanet, G.; Mota, A.J.; Colacio, E.; Triki, S. One-Dimensional Thiocyanato-Bridged Fe(II) Spin Crossover Cooperative Polymer With Unusual FeN5S Coordination Sphere. Inorg. Chem 2018, 57, 12338–12346. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, H.-Q.; Wu, G.-H.; Hou, X.-F.; Yang, J.-H.; Liu, B. Mixed-valent diruthenium diphosphonate containing zigzag chain structure of {Ru2(hedp)2(SCN)}n4n−. Inorg. Chem. Commun. 2014, 46, 241–243. [Google Scholar] [CrossRef]
- Barral, M.C.; González-Prieto, R.; Herrero, S.; Jiménez-Aparicio, R.; Priego, J.L.; Royer, E.C.; Torres, M.R.; Urbanos, F.A. Synthesis and crystal structure of high and low spin diruthenium complexes with diphenylformamidine and diphenyltriazene ligands. Polyhedron 2004, 23, 2637–2644. [Google Scholar] [CrossRef]
- Nguyen, M.; Phan, T.; Van Caemelbecke, E.; Wei, X.; Bear, J.L.; Kadish, K.M. Synthesis and Characterization of (3,1) Ru2(F3ap)4(NCS) and (3,1) Ru2(F3ap)3(F2Oap)(NCS) Where F3ap Is the 2-(2,4,6-Trifluoroanilino)pyridinate Anion. Inorg. Chem. 2008, 47, 4392–4400. [Google Scholar] [CrossRef] [PubMed]
- Miskowski, V.M.; Loehr, T.M.; Gray, H.B. Electronic and vibrational spectra of Ru2(carboxylate)4+ complexes. Characterization of a high-spin metal-metal ground state. Inorg. Chem. 1987, 26, 1098–1108. [Google Scholar] [CrossRef]
- Miskowski, V.M.; Gray, H.B. Electronic spectra of Ru2(carboxylate)4+ complexes. Higher energy electronic excited states. Inorg. Chem. 1988, 27, 2501–2506. [Google Scholar] [CrossRef]
- Miskowski, V.M.; Hopkins, M.D.; Winkler, J.R.; Gray, H.B. Inorganic Structure and Spectroscopy; John Wiley and Sons: New York, NY, USA, 1999. [Google Scholar]
- Castro, M.A.; Roitberg, A.E.; Cukiernik, F.D. Theoretical and Experimental Studies of Diruthenium Tetracarboxylates Structure, Spectroscopy, and Electrochemistry. Inorg. Chem. 2008, 47, 4682–4690. [Google Scholar] [CrossRef]
- Barral, M.C.; González-Prieto, R.; Jiménez-Aparicio, R.; Priego, J.L.; Torres, M.R.; Urbanos, F.A. Synthesis, Properties, and Structural Characterization of Bromo- and Iodotetracarboxylatodiruthenium(II,III) Compounds. Eur. J. Inorg. Chem. 2004, 2004, 4491–4501. [Google Scholar] [CrossRef]
- Clark, R.J.H.; Franks, M.L. Resonance Raman spectra of chlorotetra-acetato- and chlorotetrabutyrato-diruthenium. J. Chem. Soc. Dalton Trans. 1976, 1825–1828. [Google Scholar] [CrossRef]
- Clark, R.J.H.; Ferris, L.T.H. Resonance Raman, excitation profile and electronic structural studies of diruthenium tetracarboxylate complexes. Inorg. Chem. 1981, 20, 2759–2766. [Google Scholar] [CrossRef]
- Barral, M.C.; de la Fuente, I.; Jiménez-Aparicio, R.; Priego, J.L.; Torres, M.R.; Urbanos, F.A. Synthesis of diruthenium(II,III) amidate compounds. Crystal structure of [Ru2(μ-HNOCC4H3S)4(thf)2]SbF6·0.5cyclohexane. Polyhedron 2001, 20, 2537–2544. [Google Scholar] [CrossRef]
- Handa, M.; Yano, N.; Okuno, A.; Nakai, H.; Mitsumi, M.; Mikuriya, M.; Kataoka, Y. Synthesis, Structure and Magnetic and Electrochemical Properties of Tetrakis(benzamidato)diruthenium(II,III) Tetrafluoroborate. Magnetochemistry 2018, 4, 21. [Google Scholar] [CrossRef]
- Ebihara, M.; Fuma, Y. Tetra-μ-acetamidato- κ4N:Oκ4O:N-bis[aquaruthenium(II,III)](Ru-Ru) perchlorate. Acta Cryst. Sect. E 2006, 62, m2802–m2804. [Google Scholar]
- Ebihara, M.; Fuma, Y. Tetra-μ-acetamidato- κ4N:Oκ4O:N-bis[aquaruthenium(II,III)](Ru-Ru) nitrate. Acta Cryst. Sect. E 2006, 62, m2805–m2807. [Google Scholar]
- Ebihara, M.; Fuma, Y. Tetra-μ-acetamidato- κ4N:Oκ4O:N-bis[aquaruthenium(II,III)](Ru-Ru) tetraphenylborate monohydrate. Acta Cryst. Sect. E 2006, 62, m2808–m2810. [Google Scholar]
- Zhang, H.-X.; Ke, W.-S.; Zhu, C.-Y.; Wang, J.-Y.; Sasaki, Y.; Chen, Z.-N.; Lin, C.; Wang, Z.; Liao, S.; Wu, W. Synthesis, characterization and properties of oxo-bridged diruthenium(III) complexes with thiocyanato and cyanato ligands. Inorg. Chim. Acta 2018, 469, 469–477. [Google Scholar] [CrossRef]
- Norman, G.J.; Renzoni, G.E.; Case, D.A. Electronic Structure of Ru2(O2CR)4+ and Rh2(O2CR)4+ Complexes. J. Am. Chem. Soc. 1979, 101, 5256–5267. [Google Scholar] [CrossRef]
- Barral, M.C.; Jiménez-Aparicio, R.; Pérez-Quintanilla, D.; Priego, J.L.; Royer, E.C.; Torres, M.R.; Urbanos, F.A. Magnetic Properties of Diruthenium(II,III) Carboxylate Compounds. Crystal Structures of Ru2Cl(μ-O2CCHCHCHCHMe)4 and Ru2Cl(μ-O2CCH2OMe)4. Inorg. Chem. 2000, 39, 65–70. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yoshioka, D.; Handa, M. Magnetic interactions in one-, two-, and three-dimensional assemblies of dinuclear ruthenium carboxylates. Coord. Chem. Rev. 2006, 250, 2194–2211. [Google Scholar] [CrossRef]
- Cukiernik, F.D.; Luneau, D.; Marchon, J.-C.; Maldivi, P. Mixed-Valent Diruthenium Long-Chain Carboxylates. 2. Magnetic Properties. Inorg. Chem. 1998, 37, 3698–3704. [Google Scholar] [CrossRef]
- Khan, O. Molecular Magnetism; VCH Publisher Inc.: New York, NY, USA, 1993. [Google Scholar]



| Compound | Method | T/°C | treaction + tcooling | Yield/% |
|---|---|---|---|---|
| 2 | Conventional | r.t. | 48 h + 0 | 24 |
| Solvothermal | 80 | 24 h + 16 h | 22 | |
| Microwave | 80 | 16 h + 20 min | 33 | |
| 3 | Conventional | r.t. | 48 h + 0 | 44 |
| Solvothermal | 80 | 24 h + 16 h | 54 | |
| Microwave | 80 | 16 h + 20 min | 33 | |
| 4 | Conventional | r.t. | 48 h + 0 | 66 |
| Solvothermal | 80 | 24 h + 16 h | 63 | |
| Microwave | 80 | 16 h + 20 min | 78 |
| Compound | σ(Laxial) → σ*(Ru2) | π(O/N) → π*(Ru2) | π(RuO/N,Ru2) → π*(Ru2) | σ(Ru-Laxial) → π*(Ru2) | δ(Ru2) → δ*(Ru2) |
|---|---|---|---|---|---|
| 2 | 269 | 352 | 466 | 598sh | 967 |
| 3 | 314 | 360sh | 476 | 592sh | 1000 |
| 4 | 299 | 362sh | 475 | 592sh | 989 |
| Compound | µeff. (R.T., µB) | g | D (cm−1) | zJ (cm−1) | TIP (emu/mol) | P(%) | σ2 |
|---|---|---|---|---|---|---|---|
| 2 | 3.97 | 2.05 | 54.57 | −0.93 | 3.71 × 10−4 | 6.29 × 10−5 | 3.84 × 10−4 |
| 3 | 4.37 | 2.16 | 62.72 | −0.79 | 8.51 × 10−4 | 5.06 × 10−5 | 1.58 × 10−5 |
| 4 | 3.90 | 2.02 | 43.00 | −1.11 | 1.48 × 10−4 | 2.97 × 10−5 | 7.88 × 10−5 |
| σ2 = Σ(μeff. calcd. − μeff. exp.)2/Σμeff. exp.2 | |||||||
| 1 | 2 | 3 | |
|---|---|---|---|
| Formula | C40H48BF4N4O6Ru2 | C33H32N5O4Ru2S | C33H32N5O4Ru2S |
| fw | 969.77 | 796.83 | 796.83 |
| Space group | P | P | P |
| a/Å | 10.4084(8) | 11.4616(15) | 11.158(3) |
| b/Å | 10.90758(3) | 11.6233(15) | 12.746(3) |
| c/Å | 11.2986(9) | 14.6351(19) | 14.768(3) |
| α/° | 67.0540(10) | 99.536(2) | 66.649(4) |
| β/° | 67.4970(10) | 106.323(2) | 75.001(4) |
| γ/° | 71.4570(10) | 106.346(2) | 69.244(4) |
| V/Å3 | 1069.59(14) | 1730.9(4) | 1785.9(7) |
| Z | 1 | 2 | 2 |
| d calc/g·cm−3 | 1.506 | 1.529 | 1.482 |
| μ/mm−1 | 0.772 | 0.975 | 0.945 |
| R indices (I ≥ 2σ (I)]) | R1 = 0.0627 wR2 = 0.1934 | R1 = 0.0671 wR2 = 0.1607 | R1 = 0.0735 wR2 = 0.2081 |
| GooF on F2 | 1.092 | 1.027 | 1.010 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Da Silva, S.; Delgado-Martínez, P.; Cortijo, M.; González-Prieto, R.; Priego, J.L.; Herrero, S.; Jiménez-Aparicio, R. Magnetostructural Studies on Zigzag One-Dimensional Coordination Polymers Formed by Tetraamidatodiruthenium(II,III) Paddlewheel Units Bridged by SCN Ligands. Magnetochemistry 2019, 5, 40. https://doi.org/10.3390/magnetochemistry5030040
Moreno-Da Silva S, Delgado-Martínez P, Cortijo M, González-Prieto R, Priego JL, Herrero S, Jiménez-Aparicio R. Magnetostructural Studies on Zigzag One-Dimensional Coordination Polymers Formed by Tetraamidatodiruthenium(II,III) Paddlewheel Units Bridged by SCN Ligands. Magnetochemistry. 2019; 5(3):40. https://doi.org/10.3390/magnetochemistry5030040
Chicago/Turabian StyleMoreno-Da Silva, Sara, Patricia Delgado-Martínez, Miguel Cortijo, Rodrigo González-Prieto, José Luis Priego, Santiago Herrero, and Reyes Jiménez-Aparicio. 2019. "Magnetostructural Studies on Zigzag One-Dimensional Coordination Polymers Formed by Tetraamidatodiruthenium(II,III) Paddlewheel Units Bridged by SCN Ligands" Magnetochemistry 5, no. 3: 40. https://doi.org/10.3390/magnetochemistry5030040
APA StyleMoreno-Da Silva, S., Delgado-Martínez, P., Cortijo, M., González-Prieto, R., Priego, J. L., Herrero, S., & Jiménez-Aparicio, R. (2019). Magnetostructural Studies on Zigzag One-Dimensional Coordination Polymers Formed by Tetraamidatodiruthenium(II,III) Paddlewheel Units Bridged by SCN Ligands. Magnetochemistry, 5(3), 40. https://doi.org/10.3390/magnetochemistry5030040