Structure, DFT Calculations, and Magnetic Characterization of Coordination Polymers of Bridged Dicyanamido-Metal(II) Complexes
Abstract
:1. Introduction
2. Discussion of the Results
2.1. Preparation and Spectroscopy (IR, UV/Vis) of Complexes
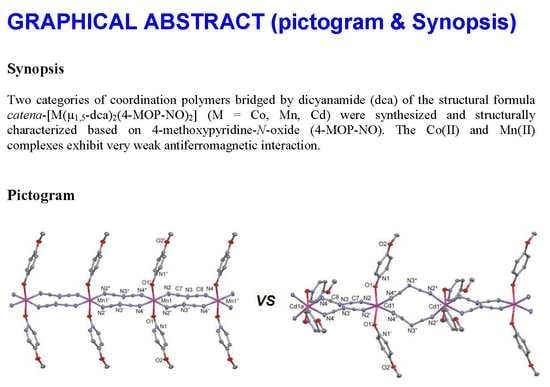
2.2. Crystal Structures
2.3. Magnetic Data
3. DFT Calculations
4. Conclusions
5. Experimental
5.1. Materials and Physical Measurements
5.2. Preparation of the Compounds
5.2.1. Preparation of Catena-[M(μ1,5-dca)2(4-MOP–NO)2] (1–3)
5.2.2. Synthesis of [Cu(dca)2(4-MOP-NO)2] (4)
5.3. X-Ray Single Crystal Measurements of 1–4
5.4. The Computational Methodology
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, S.-H.; Liu, L.-J.; Ju, H.-Y.; Liu, X.-Y.; Li, Y.-G.; Yan, S.-P. Dicyanamide bridged Cu(II)-metallacrown-6 complex with 1,4,7-triisopropyl-1,4,7-triaza-cyclononane and binding properties with DNA. Molecules 2018, 23, 1269. [Google Scholar] [CrossRef]
- Mautner, F.A.; Traber, M.; Jantscher, P.; Fischer, R.C.; Reichmann, K.; Vicente, R.; Arafat, N.; Massoud, S.S. Thiocyanato-metal(II) and azido-cobalt(III) complexes with hydroxymethylpyridines. Polyhedron 2019, 161, 309–316. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Torvisco, A.; Henary, M.M.; Milner, A.; DeVillier, H.; Karsili, T.N.V.; Louka, F.R.; Massoud, S.S. Steric effects of alkyl substituents at N-donor bidentate amines direct the nuclearity, bonding and bridging modes in isothiocyanato-copper(II) coordination compounds. Crystals 2019, 9, 38. [Google Scholar] [CrossRef]
- Mautner, F.A.; Traber, M.; Fischer, R.C.; Torvisco, A.; Reichmann, K.; Speed, S.; Vicente, R.; Massoud, S.S. Synthesis and structural characterization of thiocyanato-4-methoxypyridine-cobalt(II) complexes with diverse geometries and a bridged 1D coordination polymer showing metamagnetic transition. Polyhedron 2018, 154, 436–442. [Google Scholar] [CrossRef]
- Massoud, S.S.; Henary, M.M.; Maxwell, L.; Martín, A.; Ruiz, E.; Vicente, R.; Fischer, R.C.; Mautner, F.A. Structure, magnetic properties and DFT calculations of azido-copper(II) complexes with different azido-bonding, nuclearity and dimensionality. New J. Chem. 2018, 42, 2627–2639. [Google Scholar] [CrossRef]
- Wöhlert, S.; Ruschewitz, U.; Näther, C. Metamagnetism and slow relaxation of the magnetization in the 2D coordination polymer: [Co(NCSe)2(1,2-bis(4-pyridyl)ethylene)]n. Cryst. Growth Des. 2018, 12, 2715–2718. [Google Scholar] [CrossRef]
- Mautner, F.A.; Berger, C.; Fischer, R.C.; Massoud, S.S.; Vicente, R. Synthesis, structural characterization and magnetic properties of polymeric azido Mn(II) complexes based on methylpyridine-N-oxide co-ligands. Polyhedron 2017, 134, 126–134. [Google Scholar] [CrossRef]
- Escuer, A.; Esteban, J.; Perlepes, S.P.; Stamatatos, T.C. The bridging azido ligand as a central “player” in high-nuclearity 3d-metal cluster chemistry. Coord. Chem. Rev. 2014, 275, 87–129. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Reichmann, K.; Gullett, E.; Ashkar, K.; Massoud, S.S. Synthesis and characterization of 1D and 2D cadmium(II)-2,2’-bipyridine-N,N’-dioxide coordination polymers bridged by pseudohalides. J. Mol. Struct. 2019, 1175, 797–803. [Google Scholar] [CrossRef]
- Mautner, F.A.; Traber, M.; Fischer, R.C.; Massoud, S.S.; Vicente, R. Synthesis, crystal structures, spectral and magnetic properties of 1-D polymeric dicyanamido metal(II) complexes. Polyhedron 2017, 138, 13–20. [Google Scholar] [CrossRef]
- Massoud, S.S.; Lemieux, M.C.; Le Quan, L.L.; Vicente, R.; Albering, J.H.; Mautner, F.A. Dicyanamido-metal(II) complexes. Part 6: 1-D polymeric copper(II) complexes bridging by dicyanamide. Effect of copper(II) salt on the nature of the polymeric product. Inorg. Chim. Acta 2012, 388, 71–77. [Google Scholar] [CrossRef]
- Mautner, F.A.; Albering, J.H.; Mikuriya, M.; Massoud, S.S. Dicyanamido-metal(II) complexes. Part 5: First example for a unit cell containing dinuclear and 1-D polymeric Cu(II) complexes bridging by dicyanamide. Inorg. Chem. Commun. 2010, 13, 796–799. [Google Scholar] [CrossRef]
- Mautner, F.A.; Mikuriya, M.; Ishida, H.; Louka, F.R.; Humphrey, J.W.; Massoud, S.S. Dicyanamido-metal(II) complexes. Part 4. Synthesis, structure and magnetic characterization of polynuclear Cu(II) and Ni(II) complexes bridged by μ-1,5-dicyanamide. Inorg. Chim. Acta 2009, 362, 4073–4080. [Google Scholar] [CrossRef]
- Batten, R.S.; Murray, K.S. Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- Chakraborty, P.; Mondal, S.; Das, S.; Jana, A.D.; Das, D. Dicyanamide mediated construction of 1D polymeric networks of quinoxaline with d10 metal ions: Synthesis, thermogravimetric analysis, photoluminescence and a theoretical investigation on the π-π interactions. Polyhedron 2014, 70, 11–19. [Google Scholar] [CrossRef]
- Banerjee, S.; Halder, S.; Brandao, P.; Gomez Garcia, C.J.; Benmansour, S.; Saha, A. Synthesis and characterization of a novel dicyanamide-bridged Co(II) 1-D coordination polymer with a N4-donor Schiff base ligand. Inorg. Chim. Acta 2017, 464, 65–73. [Google Scholar] [CrossRef]
- Zheng, L.L.; Zhou, C.X.; Hu, S.; Zhou, A.J. Structural diversification of coordination assemblies of MII-dca–hydroxylpyridine (dca = dicyanamide). Polyhedron 2016, 104, 91–98. [Google Scholar] [CrossRef]
- Jensen, P.; Batten, S.R.; Moubaraki, B.; Murray, K.S. Infinite molecular tubes: Structure and magnetism of M(dca)2(apym) [M = Co, Ni, apym = 2-aminopyrimidine, dca = dicyanamide, N(CN)2−]. Chem. Commun. 2000, 9, 793–794. [Google Scholar] [CrossRef]
- Jensen, P.; Price, D.J.; Batten, S.R.; Moubaraki, B.; Murray, K.S. Self-penetration—A structural compromise between single Networks and interpenetration: Magnetic properties and crystal structures of [Mn(dca)2(H2O)] and [M(dca)(tcm)], M=Co, Ni, Cu, dc a= Dicyanamide, N(CN)2−, tcm = tricyanomethanide, C(CN)3−. Chem. Eur. J. 2000, 6, 3186–3195. [Google Scholar] [CrossRef]
- Zheng, L.-L.; Leng, J.-D.; Liu, W.-T.; Zhang, W.-X.; Lu, J.-X.; Tong, M.-L. Cu2+-Mediated nucleophilic addition of different nucleophiles to dicyanamide—Synthesis, structures, and magnetic properties of a family of mononuclear, trinuclear, hexanuclear, and polymeric copper(II) complexes. Eur. J. Inorg. Chem. 2008, 2008, 4616–4624. [Google Scholar] [CrossRef]
- Vangdal, B.; Carranza, C.; Lloret, F.; Julve, M.; Sletten, J.J. Syntheses, crystal structures and magnetic properties of copper(II) dicyanamide complexes; dinuclear, chain and ladder compounds. Chem. Soc. Dalton Trans. 2002, 4, 566–574. [Google Scholar] [CrossRef]
- Genre, C.; Jeanneau, E.; Bousseksou, A.; Luneau, D.; Borshch, S.A.; Galina, S.; Matouzenko, G.S. First dicyanamide-bridged spin-crossover coordination polymer: Synthesis, structural, magnetic, and spectroscopic studies. Chem. A Eur. J. 2008, 40, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Carranza, J.C.; Brennan, C.; Sletten, J.; Lloret, F.; Julve, M.J. Three one-dimensional systems with end-to-end dicyanamide bridges between copper(II) centres: Structural and magnetic properties. Chem. Soc. Dalton Trans. 2002, 16, 3164–3170. [Google Scholar] [CrossRef]
- Armentano, D.; De Munno, G.; Guerra, F.; Julve, M.; Lloret, F. Ligand effects on the structures of extended networks of dicyanamide-containing transition-metal ions. Inorg. Chem. 2006, 45, 4626–4636. [Google Scholar] [CrossRef] [PubMed]
- Van Albada, G.A.; van der Horst, M.G.; Teat, S.J.; Gamez, P.; Roubeau, O.; Mutikainen, I.; Turpeinen, U.; Reedijk, J. Polynuclear Cu(II), Ni(II) and Cd(II) coordination compounds with bis(pyrimidin-2-yl)amine and dicyanamide. Polyhedron 2009, 28, 1541–1545. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, T.; Das, S.; Mondal, S.; Suresh, E.; Zangrando, E.; Das, D. Thiocyanate and dicyanamide anion controlled nuclearity in Mn, Co, Ni, Cu, and Zn metal complexes with hemilabile ligand 2-benzoylpyridine. Cryst. Growth Des. 2011, 11, 3198–3205. [Google Scholar] [CrossRef]
- Halder, G.J.; Kepert, C.J.; Moubaraki, B.; Murray, K.S.; Cashion, J.D. Guest-dependent spin crossover in a nanoporous molecular framework material. Science 2002, 298, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.N.; Huang, Y.L.; Jiang, L.; Lu, T.-B. A luminescent microporous metal–organic framework with highly selective CO2 adsorption and sensing of nitro explosives. Inorg. Chem. 2014, 53, 9457–9459. [Google Scholar] [CrossRef]
- Getman, R.B.; Bae, Y.-S.; Wilmer, C.E.; Snurr, R.Q. Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal–organic frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Ranganathan, A.; Pedireddi, V.R.; Raju, A.R. A novel hybrid layer compound containing silver sheets and an organic spacer. Chem. Commun. 2000, 1, 39–40. [Google Scholar] [CrossRef]
- Ruben, M.; Lehn, J.-M.; Vaughan, G. Synthesis of ionisable [2 × 2] grid-type metallo-arrays and reversible protonic modulation of the optical properties of the [CoII4L4]8+ species. Chem. Commun. 2003, 12, 1338–1339. [Google Scholar] [CrossRef]
- Ivanova, B.; Spiteller, M. AgI and ZnII complexes with possible application as NLO materials—Crystal structures and properties. Polyhedron 2011, 30, 241–245. [Google Scholar] [CrossRef]
- Ma, L.-F.; Wang, L.-Y.; Wang, Y.-Y.; Batten, S.R.; Wang, J.-G. Self-assembly of a series of cobalt(II) coordination polymers constructed from H2tbip and dipyridyl-based ligands. Inorg. Chem. 2009, 48, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Li, H.; Su, J.; Fu, X.; Yang, B.; Gu, W.; Liu, X. Zinc metal–organic framework for selective detection and differentiation of Fe(III) and Cr(VI) ions in aqueous solution. Inorg. Chem. 2017, 56, 12348–12356. [Google Scholar] [CrossRef] [PubMed]
- Mal, D.; Sen, R.; Brandao, P.; Lin, Z. Crystallization of five new supramolecular networks with both bipyridyl and dicyanamide ligands. Polyhedron 2013, 53, 249–257. [Google Scholar] [CrossRef]
- Mautner, F.A.; Berger, C.; Fischer, R.C.; Massoud, S.S. Synthesis, characterization and luminescence properties of zinc(II) and cadmium(II) pseudohalide complexes derived from quinoline-N-oxide. Inorg. Chim. Acta 2016, 439, 69–76. [Google Scholar] [CrossRef]
- Evans, O.R.; Lin, W. Crystal engineering of NLO materials based on metal−organic coordination networks. Acc. Chem. Res. 2002, 35, 511–522. [Google Scholar] [CrossRef]
- Kahn, O. Chemistry and physics of supramolecular magnetic materials. Acc. Chem. Res. 2000, 33, 647–657. [Google Scholar] [CrossRef]
- Yang, G.-S.; Liu, C.B.; Liu, H.; Robbins, J.; Zhang, Z.J.; Yin, H.S.; Wen, H.-L.; Wang, Y.-H. Rational assembly of Pb(II)/Cd(II)/Mn(II) coordination polymers based on flexible V-shaped dicarboxylate ligand: Syntheses, helical structures and properties. J. Solid State Chem. 2015, 225, 391–401. [Google Scholar] [CrossRef]
- Qiao, J.-Z.; Zhan, M.-S.; Hu, T.-P. Syntheses, crystal structures, photoluminescent/magnetic properties of four new coordination polymers based on 2,3′,4,5′-biphenyltetracarboxylic acid. RSC Adv. 2014, 4, 62285–62294. [Google Scholar] [CrossRef]
- Mautner, F.A.; Soileau, J.B.; Bankole, P.K.; Gallo, A.; Massoud, S.S. Synthesis and spectroscopic characterization of dicyanamido-Cu(II) complexes. Part 2. Crystal structure of the complexes of tris[2-(2-pyridylethyl)]amine, tris(2-pyridylmethyl)amine and 1,4-bis[2-(2-pyridylethyl)]-piperazine. J. Mol. Struct. 2008, 889, 271–278. [Google Scholar] [CrossRef]
- Sun, H.-L.; Gao, S.; Ma, B.-Q.; Su, G. Long-range ferromagnetic ordering in two-dimensional coordination polymers Co[N(CN)2]2(L) [L = pyrazine dioxide (pzdo) and 2-methyl pyrazine dioxide (mpdo)] with dual μ- and μ3-[N(CN)2] bridges. Inorg. Chem. 2003, 42, 5399–5404. [Google Scholar] [CrossRef] [PubMed]
- Costes, J.-P.; Novitchi, G.; Shova, S.; Dahan, F.; Donnadieu, B.; Tuchagues, J.-P. Synthesis, structure, and magnetic properties of heterometallic dicyanamide-bridged Cu−Na and Cu−Gd one-dimensional polymers. Inorg. Chem. 2004, 43, 7792–7799. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.-C.; Liu, Z.-Y.; Chen, S.-H.; Su, Y.-H.; Zhang, Y.-Y.; Zhao, X.-J. Four linear CuII3 subunit-based coordination polymers with various inter-subunit connections, spin ground-states and intra-/inter-subunit magnetic couplings. Dalton Trans. 2015, 44, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- El Fallah, M.S.; Badyine, F.; Vicente, R.; Escuer, A.; Solans, X.; Font-Bardia, M. Complementarity and countercomplementarity in polynuclear copper(II) complexes with R2NCH2CH(OH)CH2NR2 (R = H, CH3): Crystal structures and magnetic study. Dalton Trans. 2002, 2934–2942. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Jensen, P.; Moubaraki, B.; Murray, K.S. Anionic metal dicyanamide networks with paramagnetic counter-cations. Chem. Commun. 2000, 23, 2331–2332. [Google Scholar] [CrossRef]
- Miller, J.S.; Manson, J. Designer magnets containing cyanides and nitriles. Acc. Chem. Res. 2001, 34, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.L.; Huang, Q.-Z.; Lynn, J.W.; Koo, H.-J.; Whangbo, M.H.; Bateman, R.; Otsuka, T.; Wada, N.; Argyriou, D.N.; Miller, J.S. Long-range magnetic order in Mn [N(CN)2]2(pyz) {pyz = pyrazine}. Susceptibility, magnetization, specific heat, and neutron diffraction measurements and electronic structure calculations. J. Am. Chem. Soc. 2001, 123, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, J.A.; Manson, J.L.; Geiser, U. Structural and magnetic diversity in tetraalkylammonium salts of anionic M[N(CN)2]3− (M = Mn and Ni). Three-dimensional coordination polymers. Inorg. Chem. 2005, 44, 3194–3202. [Google Scholar]
- Kutasi, A.M.; Batten, S.R.; Moubaraki, B.; Murray, K.S. New 2D coordination polymers containing both bi- and tri-dentate dicyanamide bridges and intercalated phenazine. J. Chem. Soc. Dalton Trans. 2002, 6, 819–821. [Google Scholar] [CrossRef]
- Manson, J.L.; Gu, J.; Wang, H.-H.; Schlueter, J.A. Structures and magnetic behavior of 1-, 2-, and 3D coordination polymers in the Cu(II)−dicyanamide−pyrimidine family. Inorg. Chem. 2003, 42, 3950–3955. [Google Scholar] [CrossRef] [PubMed]
- Talukder, P.; Shit, S.; Sasmal, A.; Batten, S.R.; Moubaraki, B.; Murray, K.S.; Mitra, S. An antiferromagnetically coupled hexanuclear copper(II) Schiff base complex containing phenoxo and dicyanamido bridges: Structural aspects and magnetic properties. Polyhedron 2011, 30, 1767–1773. [Google Scholar] [CrossRef]
- Sen, R.; Bhattacharjee, A.; Gutlich, P.; Miyashita, Y.; Okamoto, K.-I.; Koner, S. Structural and magnetic diversity in metal-dicyanamido polymer moieties: Paramagnetic and antiferromagnetic 1D chain compound and weakly ferromagnetic 2D motif. Inorg. Chim. Acta 2009, 362, 4663–4670. [Google Scholar] [CrossRef]
- Mautner, F.A.; Berger, C.; Fischer, R.C.; Massoud, S.S.; Vicente, R. Synthesis, structural characterization and magnetic properties of Mn(II) isothiocyanate complexes based on pyridine-N-oxide derivative co-ligands. Polyhedron 2018, 141, 17–24. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Ma, Y.-T.; Bao, Q.-Q.; Sun, B.-W. Syntheses, crystal structure and properties of two 1-D coordination polymers bridged by dicyanamides. J. Chem. Crystallogr. 2012, 42, 628–632. [Google Scholar] [CrossRef]
- Sun, B.-W.; Gao, S.; Ma, B.-Q.; Niu, D.-Z.; Wang, Z.-M. Syntheses, structures and magnetic properties of three-dimensional co-ordination polymers constructed by dimer subunits. J. Chem. Soc. Dalton Trans. 2000, 22, 4187–4191. [Google Scholar] [CrossRef]
- Ghoshal, D.; Mostafa, G.; Maji, T.K.; Zangrando, E.; Lu, T.-H.; Ribas, J.; Chaudhuri, N.R. Synthesis, crystal structure and magnetic behavior of three polynuclear complexes: [Co(pyo)2(dca)2]n, [Co3(ac)4(bpe)3(dca)2]n and [{Co(male)(H2O)2}(H2O)]n [pyo, pyridine-N-oxide; dca, dicyanamide; ac, acetate; bpe, 1,2-bis-(4-pyridyl)ethane and male, maleate]. New J. Chem. 2004, 28, 1204–1213. [Google Scholar]
- Van Albada, G.A.; Mutikainen, I.; Turpeinen, U.; Reedijk, J. A rare 2D structure of a novel Cu(II) dinuclear-based compound with dicyanamide and 4-nitropyridine-N-oxide as ligands. Inorg. Chem. Commun. 2006, 9, 441–443. [Google Scholar] [CrossRef]
- Hnatejko, Z.; Kwiatek, D.; Durkiewicz, G.; Kubicki, M.; Jastrazab, R.; Lis, S. Pyridine N-oxide complexes of Cu(II) ions with pseudohalides: Synthesis, structural and spectroscopic characterization. Polyhedron 2014, 81, 728–734. [Google Scholar] [CrossRef]
- Manson, J.L.; Lee, D.W.; Rheingold, A.L.; Miller, J.S. Buckled-layered Structure of Zinc Dicyanamid, ZnII[N(CN)2]2. Inorg. Chem. 1998, 37, 5966–5967. [Google Scholar] [CrossRef]
- Köhler, H.; Kolbe, A.; Lux, G.Z. Metal-pseudohalogenide 27. Zur struktur der dicyanamide zweiwertiger 3d-metalle M(N(CN)2)2. Anorg. Allg. Chem. 1977, 428, 103–112. [Google Scholar] [CrossRef]
- Hathaway, B.J. Comprehensive Coordination Chemistry; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; Volume 5, p. 533. [Google Scholar]
- Mautner, F.A.; Jantscher, P.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Karsili, T.N.V.; Massoud, S.S. Synthesis and characterization of 1D coordination polymers of metal(II)-dicyanamido complexes. Polyhedron 2019, 166, 34–43. [Google Scholar] [CrossRef]
- Claramunt, A.; Escuer, A.; Mautner, F.A.; Sanz, N.; Vicente, R.J. Two new one-dimensional systems with end-to-end single dicyanamide bridges between manganese(II) centres: Structural and magnetic properties. Chem. Soc. Dalton Trans. 2000, 15, 2627–2630. [Google Scholar] [CrossRef]
- Manson, J.L.; Schlueter, J.A.; Nygren, C.L. Mn(dca)2(pym)2 and Mn(dca)2(pym)(H2O) {dca = dicyanamide; pym = pyrimidine}: New coordination polymers exhibiting 1- and 2-D topologies. Dalton Trans. 2007, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Wriedt, M.; Näther, C. Directed synthesis of μ-1,3,5 bridged dicyanamides by thermal decomposition of μ-1,5 bridged precursor compounds. Dalton Trans. 2011, 40, 886–898. [Google Scholar] [CrossRef] [PubMed]
- De la Pinta, N.; Martín, S.; Urtiaga, M.K.; Barandika, M.G.; Arriortua, M.I.; Lezama, I.; Madariaga, G.; Cortés, R. Structural analysis, spectroscopic, and magnetic properties of the 1D triple-bridged compounds [M(dca)2(bpa)] (M = Mn, Fe, Co, Zn; dca = dicyanamide; bpa = 1,2-bis(4-pyridyl)ethane) and the 3D [Ni(dca)(bpa)2]dca·6H2O. Inorg. Chem. 2010, 49, 10445–10454. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Marschner, C.; Cano, J.; Baumgartner, J.; Ribas, J.; El Fallah, M.S.; Mitra, S. Synthesis, crystal structures and magnetic behaviors of two dicyanamide bridged di- and polynuclear complexes of cobalt(II) derived from 2,4,6-tris(2-pyridyl)1,3,5-triazine and imidazole. Polyhedron 2009, 28, 2436–2442. [Google Scholar] [CrossRef]
- Fisher, M.E. Perpendicular susceptibility of the Ising model. J. Math. Phys. 1963, 4, 124–135. [Google Scholar] [CrossRef]
- Fisher, M.E. Magnetism in one-dimensional systems-the Heisenberg model for infinite spin. Am. J. Phys. 1964, 32, 343–346. [Google Scholar] [CrossRef]
- Escuer, A.; Mautner, F.A.; Sanz, N.; Vicente, R. Syntheses, structures and magnetic properties of the dicyanamide (dca) polynuclear compounds [Mn(ac)(terpy)(μ1,5-dca)]n, [Mn(pdz)2(μ1,5-dca)2]n and [{Mn(dca)(terpy)-(MeOH)}2(μ-terephthalate)]. Inorg. Chim. Acta 2002, 340, 163–169. [Google Scholar] [CrossRef]
- Biswas, M.; Rosair, G.M.; Pilet, G.M.; Mitra, S. Syntheses, structures and magnetic properties of [MII(dca)2(CH3OH)2]n, where M = Co or Cu and dca = dicyanamide, N(CN)2−. Inorg. Chim. Acta 2007, 360, 695–699. [Google Scholar] [CrossRef]
- Van Albada, G.A.; Quiroz-Castro, M.E.; Mutikainen, I.; Turpeinen, U.; Reedijk, J. The first structural evidence of a polymeric Cu(II) compound with a bridging dicyanamide anion: X-ray structure, spectroscopy and magnetism of catena-[polybis(2-aminopyrimidine)copper(II)bis(μ-dicyanamido)]. Inorg. Chim. Acta 2000, 298, 221–225. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Zhang, J. An efficient strategy for self-assembly of DNA-mimic homochiral 1D helical Cu(II) chain from achiral flexible ligand by spontaneous resolution. Inorg. Chem. 2016, 55, 3378–3383. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.B.; Cui, A.L.; Kou, H.Z. Assembly of dinuclear copper(II) secondary building units into polymeric complexes: Crystal structures and magnetic properties. CrystEngComm 2014, 16, 8027–8034. [Google Scholar] [CrossRef]
- Escuer, A.; Mautner, F.A.; Sanz, N.; Vicente, R. Two new one-dimensional compounds with end-to-end dicyanamide as a bridging ligand: syntheses and structural characterization of trans-[Mn(4-bzpy)2(N(CN)2)2]n and cis-[Mn(Bpy)(N(CN)2)2]n, (4-bzpy = 4-benzoylpyridine; bpy = 2,2‘-bipyridyl). Inorg. Chem. 2000, 39, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- Bruker APEX, SAINT v. 8.37A; Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. SADABS v. 2; University of Goettingen: Goettingen, Germany, 2001. [Google Scholar]
- Sheldrick, G.M. A Short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, T.; van de Streek, J.J. Mercury: Visualization and analysis of crystal structures. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON, a Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 1999. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]














| Comp. | Ring(I)…Ring(J) | Symmetry of J | Cg…Cg (Å) | Alpha (°) | Cg(I)_perp (Å) |
|---|---|---|---|---|---|
| 1 | Cg(py)...Cg(py) | [1-x,-1/2+y,1/2-z] | 4.7136(8) | 1.85(6) | 3.2119(5) |
| Cg(py)...Cg(py) | [1-x,1/2+y,1/2-z] | 4.7135(8) | 1.85(6) | 3.2118(5) | |
| 2 | Cg(py)...Cg(py) | [3/2-x,-1/2+y,2-z] | 4.7115(12) | 2.57(10) | 3.3814(9) |
| Cg(py)...Cg(py) | [3/2-x,1/2+y,2-z] | 4.7114(12) | 2.57(10) | 3.2329(9) | |
| 3 | Cg(py)...Cg(py) | [1-x,-1/2+y,3/2-z] | 4.6548(8) | 0.61(6) | 3.2300(5) |
| Cg(py)...Cg(py) | [1-x,1/2+y,3/2-z] | 4.6548(8) | 0.61(6) | 3.2652(5) |
| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C16H14CoN8O4 | C16H14MnN8O4 | C16H14CdN8O4 | C16H14CuN8O4 |
| Formula mass | 441.28 | 437.29 | 494.76 | 445.90 |
| System | Monoclinic | Orthorhombic | Orthorhombic | Monoclinic |
| Space group | P21/c | P21212 | Pccn | P21/c |
| a (Å) | 7.3603(4) | 18.2240(7) | 17.7537(7) | 11.0685(5) |
| b (Å) | 6.6096(5) | 6.6283(3) | 6.6690(3) | 6.0407(2) |
| c (Å) | 18.4806(13) | 7.5065(3) | 15.2622(6) | 13.4842(5) |
| β (°) | 101.257(4) | 90 | 90 | 91.770(2) |
| V (Å3) | 881.76(10) | 906.74(6) | 1807.04(13) | 901.14(6) |
| Z | 2 | 2 | 4 | 2 |
| T (K) | 100(2) | 100(2) | 100(2) | 100(2) |
| μ (mm−1) | 1.018 | 0.772 | 1.253 | 1.256 |
| Dcalc (Mg/m3) | 1.662 | 1.602 | 1.819 | 1.643 |
| θ max (°) | 30.598 | 30.092 | 29.998 | 30.148 |
| Data collected | 38338 | 29257 | 70221 | 48444 |
| Unique refl. | 2707 | 2662 | 2648 | 2643 |
| Rint | 0.0547 | 0.0522 | 0.0378 | 0.0306 |
| Parameters | 134 | 134 | 133 | 171 |
| GooF on F2 | 1.058 | 1.134 | 1.105 | 1.092 |
| R1 (I > 2(σI)) | 0.0282 | 0.0276 | 0.0189 | 0.0219 |
| wR2 (all data) | 0.0699 | 0.0653 | 0.0431 | 0.0619 |
| Residual extrema (e/Å3) | 0.43/−0.42 | 0.45/−0.40 | 0.41/−0.68 | 0.44/−0.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mautner, F.A.; Jantscher, P.; Fischer, R.C.; Torvisco, A.; Vicente, R.; Karsili, T.N.V.; Massoud, S.S. Structure, DFT Calculations, and Magnetic Characterization of Coordination Polymers of Bridged Dicyanamido-Metal(II) Complexes. Magnetochemistry 2019, 5, 41. https://doi.org/10.3390/magnetochemistry5030041
Mautner FA, Jantscher P, Fischer RC, Torvisco A, Vicente R, Karsili TNV, Massoud SS. Structure, DFT Calculations, and Magnetic Characterization of Coordination Polymers of Bridged Dicyanamido-Metal(II) Complexes. Magnetochemistry. 2019; 5(3):41. https://doi.org/10.3390/magnetochemistry5030041
Chicago/Turabian StyleMautner, Franz A., Patricia Jantscher, Roland C. Fischer, Ana Torvisco, Ramon Vicente, Tolga N. V. Karsili, and Salah S. Massoud. 2019. "Structure, DFT Calculations, and Magnetic Characterization of Coordination Polymers of Bridged Dicyanamido-Metal(II) Complexes" Magnetochemistry 5, no. 3: 41. https://doi.org/10.3390/magnetochemistry5030041
APA StyleMautner, F. A., Jantscher, P., Fischer, R. C., Torvisco, A., Vicente, R., Karsili, T. N. V., & Massoud, S. S. (2019). Structure, DFT Calculations, and Magnetic Characterization of Coordination Polymers of Bridged Dicyanamido-Metal(II) Complexes. Magnetochemistry, 5(3), 41. https://doi.org/10.3390/magnetochemistry5030041