The Study of Structural Features of N- and O-Derivatives of 4,5-Dihydroxyimidazolidine-2-Thione by NMR Spectroscopy and Quantum Chemical Calculations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of a Mixture of cis-, trans-4,5-Dihydroxyimidazolidine-2-Thione (1c, 1t)
2.1.2. Synthesis of a Mixture of cis- and trans-4,5-Dihydroxy-1,3-Dimethylimidazolidine-2-Thione (2c, 2t)
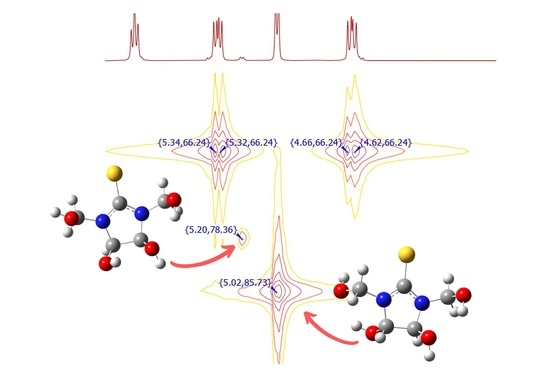
2.1.3. Synthesis of a Mixture of cis- and trans-4,5-Dihydroxy-1,3-Diphenylimidazolidine-2-Thione (4c, 4t)
2.1.4. Synthesis of a Mixture of cis- and trans-4,5-Dihydroxy-1,3-Bis(Hydroxymethyl)Imidazolidine-2-Thione (6c, 6t)
2.1.5. Synthesis of a Mixture of cis- and trans-4,5-Dimethoxyimidazolidine-2-Thione (9c, 9t)
2.1.6. Synthesis of 4,5-Diethoxyimidazolidine-2-Thione (10), 4,5-Dipropoxyimidazolidine-2-Thione (11), 4,5-Dibutoxyimidazolidine-2-Thione (12)
2.2. NMR Spectroscopy
2.3. Computational Details
3. Results and Discussion
3.1. Identification of 4,5-Dihydroxy-1,3-Bis(Hydroxymethyl)Imidazolidine-2-Thione (6)
3.2. The N-Derivatives of DHIT: General Regularities in the NMR Spectra
3.2.1. Geometric Isomerism
3.2.2. Nature of the Substituents
3.3. The O-Derivatives of DHIT: General Regularities in the NMR Spectra
Nature of Substituents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mezoughi, A.B.; Mohammed, W.A.; Ettarhouni, Z.O. Recent Biological Applications and Chemical Synthesis of Thiohydantoins. J. Chem. Rev. 2021, 3, 196–218. [Google Scholar] [CrossRef]
- Gazieva, G.A.; Anikina, L.V.; Pukhov, S.A.; Karpova, T.B.; Nelyubina, Y.V.; Kravchenko, A.N. Substituted N-aminothioglycolurils containing thiosemicarbazone moiety and their cytotoxic activity in vitro. Mol. Divers. 2016, 20, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Kim, S.H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef] [PubMed]
- Gazieva, G.A.; Anikina, L.V.; Nechaeva, T.V.; Pukhov, S.A.; Karpova, T.B.; Popkov, S.V.; Nelyubina, Y.V.; Kolotyrkina, N.G.; Kravchenko, A.N. Synthesis and biological evaluation of new substituted thioglycolurils, their analogues and derivatives. Eur. J. Med. Chem. 2017, 140, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Reany, O.; Mohite, A.; Keinan, E. Hetero-Bambusurils. Isr. J. Chem. 2018, 58, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Baranov, V.V.; Galochkin, A.A.; Nelyubina, Y.V.; Kravchenko, A.N.; Makhova, N.N. Synthesis and Structure of 1-Substituted Semithioglycolurils. Synthesis 2020, 52, 2563–2571. [Google Scholar] [CrossRef]
- Mondal, P.; Solel, E.; Fridman, N.; Keinan, E.; Reany, O. Intramolecular van der Waals Interactions Challenge Anion Binding in perthio-Bambusurils. Chem. Eur. J. 2019, 25, 13336–13343. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Solel, E.; Keinan, E.; Reany, O. Aza-Bambusurils En Route to Anion Transporters. Chem. Eur. J. 2016, 22, 8848–8854. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Mohite, A.; Deng, X.; Yang, F.; Dong, Z.; Xu, J.; Liu, J.; Keinan, E.; Reany, O. Semithiobambus[6]uril is a transmembrane anion transporter. Chem. Commun. 2017, 53, 7557–7560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Solel, E.; Keinan, E.; Reany, O. Dual-functional semithiobambusurils. Chem. Eur. J. 2015, 21, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, A.N.; Baranov, V.V.; Nelyubina, Y.V.; Gazieva, G.A.; Svitańko, I.V. Diastereoselective synthesis of 4,5-dihydroxyimidazolidin-2-ones (-thiones) and their structure. Russ. Chem. Bull. 2012, 61, 64–73. [Google Scholar] [CrossRef]
- Gazieva, G.A.; Nelyubina, Y.V.; Kravchenko, A.N.; Lyssenko, K.A.; Makhova, N.N. α-Thioureidoalkylation of urea heteroanalogs. Russ. Chem. Bull. 2009, 58, 1945–1954. [Google Scholar] [CrossRef]
- Pihtili, G.; Tuncer, H. Classical and Microwave-Assisted Synthesis of Substituted Dihydroxy-imidazolidine-2-thiones Compounds. Hacet. J. Biol. Chem. 2017, 45, 163–174. [Google Scholar] [CrossRef]
- Singh, M.; Parvari, G.; Botoshansky, M.; Keinan, E.; Reany, O. The Synthetic Challenge of Thioglycolurils. Eur. J. Org. Chem. 2014, 5, 933–940. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01.01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functional. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Nelyubina, Y.V.; Gazieva, G.A.; Baranov, V.V.; Belyakov, P.A.; Chizhov, A.O.; Lyssenko, K.A.; Kravchenko, A.N. The synthesis, structure, and electron density distribution in crystals of 4,5-dihydroxyimidazolidin-2-thiones. Russ. Chem. Bull. 2009, 58, 1353–1360. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhang, J.M.; Guo, J.P.; Qu, G.R. Trans-4,5-dihydroxyimidazolidine-2-thione. Acta Cryst. 2007, 63, 2821–2823. [Google Scholar] [CrossRef]
- Kalichkina, L.; Novikov, D.; Kotelnikov, O.; Malkov, V.; Knyazev, A. Reaction Pathway and Kinetic Study of 4,5-Dihydroxyimidazolidine-2-thione Synthesis by HPLC and NMR. Heterocycles 2022, 104, 1954–1965. [Google Scholar] [CrossRef]
- Jena, S.; Routray, C.; Dutta, J.; Biswal, H.S. Hydrogen Bonding Directed Reversal of 13 C NMR Chemical Shielding. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207521. [Google Scholar] [CrossRef] [PubMed]
- Nieuwland, C.; Fonseca Guerra, C. How the Chalcogen Atom Size Dictates the Hydrogen-Bond Donor Capability of Carboxamides, Thioamides, and Selenoamides. Chem. Eur. J. 2022, 28. [Google Scholar] [CrossRef] [PubMed]











| Compound | Designation | Substitute | 13C Chemical Shift, ppm | 1H Chemical Shift, ppm | E, a.e. | ||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | C=S | CH-CH | CH-CH | |||
 | 1c | H | H | H | H | 180.35 | 79.95 | 5.03 | –776.066928 |
| 2c | CH3 | CH3 | H | H | 181.00 | 81.67 | 4.96 | –854.664870 | |
| 3c | C2H5 | C2H5 | H | H | 179.30 | 79.40 | 5.05 | –933.276457 | |
| 4c | C6H5 | C6H5 | H | H | 180.07 | 82.60 | 5.20 | –1238.080635 | |
| 6c | CH2OH | CH2OH | H | H | 180.43 | 78.60 | 5.20 | –1005.113298 | |
| 9c | H | H | CH3 | CH3 | 183.02 | 90.93 | 4.68 | –854.634508 | |
 | 1t | H | H | H | H | 182.11 | 87.22 | 4.73 | –776.065439 |
| 2t | CH3 | CH3 | H | H | 181.54 | 87.98 | 4.73 | –854.663088 | |
| 3t | C2H5 | C2H5 | H | H | 179.60 | 86.60 | 4.80 | –933.275429 | |
| 4t | C6H5 | C6H5 | H | H | 180.15 | 89.73 | 5.61 | –1238.079358 | |
| 6t | CH2OH | CH2OH | H | H | 180.85 | 78.35 | 5.05 | –1005.116998 | |
| 9t | H | H | CH3 | CH3 | 184.03 | 91.78 | 4.83 | –854.640392 | |
| 10t | H | H | C2H5 | C2H5 | 183.77 | 90.93 | 4.73 | –933.252904 | |
| 11t | H | H | C3H7 | C3H7 | 183.68 | 90.99 | 4.73 | –1011.859766 | |
| 12t | H | H | C4H9 | C4H9 | 183.66 | 91.03 | 4.68 | –1090.466531 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalichkina, L.E.; Fateev, A.V.; Krivolapenko, P.K.; Isakova, K.A.; Knyazev, A.S.; Malkov, V.S.; Bakibaev, A.A.; Tuguldurova, V.P. The Study of Structural Features of N- and O-Derivatives of 4,5-Dihydroxyimidazolidine-2-Thione by NMR Spectroscopy and Quantum Chemical Calculations. Magnetochemistry 2023, 9, 15. https://doi.org/10.3390/magnetochemistry9010015
Kalichkina LE, Fateev AV, Krivolapenko PK, Isakova KA, Knyazev AS, Malkov VS, Bakibaev AA, Tuguldurova VP. The Study of Structural Features of N- and O-Derivatives of 4,5-Dihydroxyimidazolidine-2-Thione by NMR Spectroscopy and Quantum Chemical Calculations. Magnetochemistry. 2023; 9(1):15. https://doi.org/10.3390/magnetochemistry9010015
Chicago/Turabian StyleKalichkina, Liudmila E., Alexander V. Fateev, Polina K. Krivolapenko, Kristina A. Isakova, Alexey S. Knyazev, Victor S. Malkov, Abdigali A. Bakibaev, and Vera P. Tuguldurova. 2023. "The Study of Structural Features of N- and O-Derivatives of 4,5-Dihydroxyimidazolidine-2-Thione by NMR Spectroscopy and Quantum Chemical Calculations" Magnetochemistry 9, no. 1: 15. https://doi.org/10.3390/magnetochemistry9010015
APA StyleKalichkina, L. E., Fateev, A. V., Krivolapenko, P. K., Isakova, K. A., Knyazev, A. S., Malkov, V. S., Bakibaev, A. A., & Tuguldurova, V. P. (2023). The Study of Structural Features of N- and O-Derivatives of 4,5-Dihydroxyimidazolidine-2-Thione by NMR Spectroscopy and Quantum Chemical Calculations. Magnetochemistry, 9(1), 15. https://doi.org/10.3390/magnetochemistry9010015