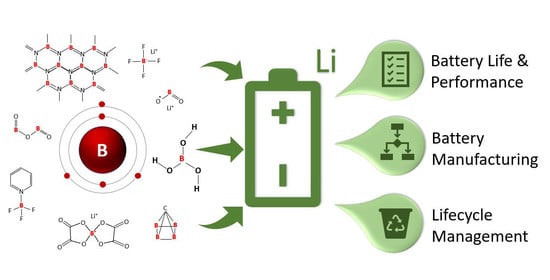
Examining the Benefits of Using Boron Compounds in Lithium Batteries: A Comprehensive Review of Literature
Abstract
:1. Introduction to Boron and Lithium
2. Boron Used in Electrolytes
2.1. Electrolyte Additives—LiBOB
2.2. Electrolyte Additives—TPFPB and TB
2.3. Electrolyte Additives—LiBF4
2.4. Solid-State Electrolyte—Boron Nitride & Boric Acid
3. Boron Used in Anodes
3.1. Graphite Anode—SEI
3.2. Graphite Anode—Graphitization
3.3. Graphite Anode—Upcycling
3.4. Graphene Anode—Li Adsorption
3.5. Other Anodes—Miscellaneous
4. Boron Used in Cathodes
4.1. LCO, LNO, and LMO
4.2. LFP
4.3. NMC
4.4. NCA and NCMA
5. Boron Used to Assist Manufacturing
6. Boron Used in Separators
7. Boron Used in BTMS
8. Boron Used in Lithium Sulfur Batteries
9. Summary and Prospect
9.1. Summary
- Various boron lithium compounds were studied and proven effective in assisting the formation and stability of SEI. These can be introduced through electrolyte additives or through direct application to the anode.
- For the direct application of boron compounds to the anode, using boric acid was reported to provide diverse benefits.
- In the cathode, boron doping and forming an LBO layer were of particular interest. Cathode doping and cathode coating are the two conventional approaches to improving the cycling performance.
- The use of boron compounds has been widely employed in experiments on those cathode systems that currently have wide commercial applications in EVs, such as NCMA, NMC, NCA, and LFP.
- Using boron compounds to assist manufacturing could be a way of achieving the “one stone, two birds” outcome. Using boron compounds to achieve multifaced benefits is common beyond batteries.
- Particularly, using boric acid was considered an effective approach in anode upcycling and cathode washing for residual lithium compounds (RLCs).
- For applications in separators and BTMS, boron nitride and its derivatives have been the primary focus of research, given their unique electrical insulation and thermal conductivity.
- For novel lithium systems such as LSBs, boron nitride in separators and boron carbide in cathodes were reported to be effective at alleviating shuttle effects through different mechanisms.
9.2. Prospect
- While there are extensive studies on coin cells, more research on pouch cells or cylindrical cells is required to narrow the significant gap towards commercialization. Gaps include but are not limited to processing difficulty and raw material costs.
- In particular, the study of using more commercially available boron sources as precursors, such as boric acid and boron oxide, will be useful to achieve a fine balance between cost and efficacy.
- With a closer link to commercialization, studies on the use of boron compounds (e.g., boric acid) to assist the actual production environment will likely attract more interest.
- The topic of battery recycling is important and currently topical in the industry. Therefore, more studies on recycling or upcycling batteries with the use of boron will be likely.
- Given the diversity of available boron compounds, using a machine learning approach and leveraging existing extensive studies on boron compound might be of interest in guiding research [153].
- Leveraging machine learning may also help with the growing need for performance benchmarking among various types of battery systems.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grew, E.S.; Hystad, G.; Toapanta, M.P.C.; Eleish, A.; Ostroverkhova, A.; Golden, J.; Hazen, R.M. Lithium mineral evolution and ecology: Comparison with boron and beryllium. Eur. J. Miner. 2019, 31, 755–774. [Google Scholar] [CrossRef] [Green Version]
- Vangioni-Flam, E.; Cassé, M.; Audouze, J. Lithium–beryllium–boron: Origin and evolution. Phys. Rep. 2000, 333, 365–387. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Wang, S.; Dewhurst, R.D.; Ignat’Ev, N.V.; Finze, M.; Braunschweig, H. Boron: Its Role in Energy-Related Processes and Applications. Angew. Chem. Int. Ed. 2019, 59, 8800–8816. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Angell, C.A. LiBOB and Its Derivatives. Electrochem. Solid-State Lett. 2001, 4, E1–E4. [Google Scholar] [CrossRef]
- Xu, K.; Lee, U.; Zhang, S.; Wood, M.; Jow, T.R. Chemical analysis of graphite/electrolyte interface formed in LiBOB-based electrolytes. Electrochem. Solid-State Lett. 2003, 6, A144. [Google Scholar] [CrossRef]
- Täubert, C.; Fleischhammer, M.; Wohlfahrt-Mehrens, M.; Wietelmann, U.; Buhrmester, T. LiBOB as electrolyte salt or additive for lithium-ion batteries based on LiNi0.8Co0.15Al0.05O2/graphite. J. Electrochem. Soc. 2010, 157, A721. [Google Scholar] [CrossRef]
- Xu, K. Tailoring Electrolyte Composition for LiBOB. J. Electrochem. Soc. 2008, 155, A733–A738. [Google Scholar] [CrossRef]
- Chen, Z.; Amine, K. Tris (pentafluorophenyl) borane as an additive to improve the power capabilities of lithium-ion batteries. J. Electrochem. Soc. 2006, 153, A1221. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, T.-K. Tris (pentafluorophenyl) borane as an electrolyte additive for LiFePO4 battery. J. Power Sources 2009, 193, 834–840. [Google Scholar] [CrossRef]
- Rong, H.; Xu, M.; Xie, B.; Liao, X.; Huang, W.; Xing, L.; Li, W. Tris (trimethylsilyl) borate (TMSB) as a cathode surface film forming additive for 5 V Li/LiNi0.5Mn1.5O4 Li-ion cells. Electrochim. Acta 2014, 147, 31–39. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lee, K.-Y.; Lee, H.-Y.; Su, Y.-H.; Her, L.-J. Trimethyl borate and triphenyl borate as electrolyte additives for LiFePO4 cathode with enhanced high temperature performance. J. Power Sources 2012, 217, 524–529. [Google Scholar] [CrossRef]
- Sun, X.; Lee, H.S.; Yang, X.-Q.; McBreen, J. Using a boron-based anion receptor additive to improve the thermal stability of LiPF6-based electrolyte for lithium batteries. Electrochem. Solid-State Lett. 2002, 5, A248. [Google Scholar] [CrossRef]
- Wang, Z.; Xing, L.; Li, J.; Li, B.; Xu, M.; Liao, Y.; Li, W. Trimethyl borate as an electrolyte additive for high potential layered cathode with concurrent improvement of rate capability and cyclic stability. Electrochim. Acta 2015, 184, 40–46. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. Low-temperature performance of Li-ion cells with a LiBF4-based electrolyte. J. Solid State Electrochem. 2003, 7, 147–151. [Google Scholar] [CrossRef]
- Shen, C.; Xiong, D.; Ellis, L.D.; Gering, K.L.; Huang, L.; Dahn, J.R. Using the charge-discharge cycling of positive electrode symmetric cells to find electrolyte/electrode combinations with minimum reactivity. J. Electrochem. Soc. 2017, 164, A3349. [Google Scholar] [CrossRef]
- Ellis, L.D.; Xia, J.; Louli, A.J.; Dahn, J.R. Effect of substituting LiBF4 for LiPF6 in high voltage lithium-ion cells containing electrolyte additives. J. Electrochem. Soc. 2016, 163, A1686. [Google Scholar] [CrossRef]
- Jow, T.; Ding, M.; Xu, K.; Zhang, S.; Allen, J.; Amine, K.; Henriksen, G. Nonaqueous electrolytes for wide-temperature-range operation of Li-ion cells. J. Power Sources 2003, 119, 343–348. [Google Scholar] [CrossRef]
- Zuo, X.; Fan, C.; Liu, J.; Xiao, X.; Wu, J.; Nan, J. Lithium tetrafluoroborate as an electrolyte additive to improve the high voltage performance of lithium-ion battery. J. Electrochem. Soc. 2013, 160, A1199. [Google Scholar] [CrossRef]
- Nie, M.; Xia, J.; Dahn, J.R. Development of pyridine-boron trifluoride electrolyte additives for lithium-ion batteries. J. Electrochem. Soc. 2015, 162, A1186. [Google Scholar] [CrossRef]
- Ma, L.; Self, J.; Nie, M.; Glazier, S.; Wang, D.Y.; Lin, Y.S.; Dahn, J.R. A systematic study of some promising electrolyte additives in Li[Ni1/3Mn1/3Co1/3]O2/graphite, Li [Ni0.5Mn0.3Co0.2]/graphite and Li [Ni0.6Mn0.2Co0.2]/graphite pouch cells. J. Power Sources 2015, 299, 130–138. [Google Scholar] [CrossRef]
- Li, M.; Zhu, W.; Zhang, P.; Yang, B.; Li, H.; He, Q.; Borisevich, A.Y.; Dai, S. Graphene-analogues Boron Nitride nanosheets with ultrahigh ionic liquid uptake capacity as quasi-solid electrolytes. Mater. Sci. 2017. preprint. Available online: https://orca.cardiff.ac.uk/92038/3/BN%2020150904.pdf (accessed on 3 April 2022).
- Jiang, H.; Wang, Z.; Ma, L.; Yang, Q.; Tang, Z.; Song, X.; Zeng, H.; Zhi, C. Boron ink assisted in situ boron nitride coatings for anti-oxidation and anti-corrosion applications. Nanotechnology 2019, 30, 335704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Antonio, R.G.; Choy, K.L. Boron nitride enhanced polymer/salt hybrid electrolytes for all-solid-state lithium ion batteries. J. Power Sources 2019, 435, 226736. [Google Scholar] [CrossRef]
- Hyun, W.J.; de Moraes, A.C.M.; Lim, J.-M.; Downing, J.R.; Park, K.-Y.; Tan, M.T.Z.; Hersam, M.C. High-Modulus Hexagonal Boron Nitride Nanoplatelet Gel Electrolytes for Solid-State Rechargeable Lithium-Ion Batteries. ACS Nano 2019, 13, 9664–9672. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.-T.F.; Kalaga, K.; Gullapalli, H.; Babu, G.; Reddy, A.L.M.; Ajayan, P.M. Hexagonal Boron Nitride-Based Electrolyte Composite for Li-Ion Battery Operation from Room Temperature to 150 °C. Adv. Energy Mater. 2016, 6, 1600218. [Google Scholar] [CrossRef]
- Wang, S.; Xu, X.; Cui, C.; Zeng, C.; Liang, J.; Fu, J.; Zhang, R.; Zhai, T.; Li, H. Air Sensitivity and Degradation Evolution of Halide Solid State Electrolytes upon Exposure. Adv. Funct. Mater. 2022, 32, 2108805. [Google Scholar] [CrossRef]
- Chen, X.; Jia, Z.; Lv, H.; Wang, C.; Zhao, N.; Guo, X. Improved stability against moisture and lithium metal by doping F into Li3InCl6. J. Power Sources 2022, 545, 231939. [Google Scholar] [CrossRef]
- Kim, D.; Liu, X.; Yu, B.; Mateti, S.; O’Dell, L.A.; Rong, Q.; Chen, Y. Amine-functionalized boron nitride nanosheets: A new functional additive for robust, flexible ion gel electrolyte with high lithium-ion transference number. Adv. Funct. Mater. 2020, 30, 1910813. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, A.; Li, N.; Li, S.; Zangiabadi, A.; Huang, W.; Li, A.C.; Jin, T.; Song, Q.; Xu, W.; et al. Stabilizing solid electrolyte-anode interface in Li-metal batteries by boron nitride-based nanocomposite coating. Joule 2019, 3, 1510–1522. [Google Scholar] [CrossRef]
- Zhang, Z.; Gonzalez, A.R.; Choy, K.L. Boron Nitride Enhanced Garnet-Type (Li6.25Al0.25La3Zr2O12) Ceramic Electrolyte for an All-Solid-State Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 7438–7448. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Sun, Z.; Gao, G.; Lu, S.; Zhu, M.; Zhang, Y.; Jia, Z.; Xiao, C.; Bu, H.; et al. Hexagonal boron nitride induces anion trapping in a polyethylene oxide based solid polymer electrolyte for lithium dendrite inhibition. J. Mater. Chem. A 2020, 8, 9579–9589. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Zhou, L.; Xu, Q.; Min, Y. Surface-modified boron nitride as a filler to achieve high thermal stability of polymer solid-state lithium-metal batteries. J. Mater. Chem. A 2021, 9, 20530–20543. [Google Scholar] [CrossRef]
- Kang, H.; Song, M.; Yang, M.; Lee, J.W. Lithium metal anode with lithium borate layer for enhanced cycling stability of lithium metal batteries. J. Power Sources 2021, 485, 229286. [Google Scholar] [CrossRef]
- Huang, Z.; Ren, J.; Zhang, W.; Xie, M.; Li, Y.; Sun, D.; Shen, Y.; Huang, Y. Protecting the Li-Metal Anode in a Li–O2 Battery by using Boric Acid as an SEI-Forming Additive. Adv. Mater. 2018, 30, 1803270. [Google Scholar] [CrossRef]
- Zhuang, D.; Huang, X.; Chen, Z.; Gong, H.; Sheng, L.; Song, L.; Wang, T.; He, J. The synergistic effect of Cu2O and boric acid forming solid electrolyte interphase layer to restrain the dendritic growth. J. Power Sources 2020, 458, 228055. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material–fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Kurita, N. Molecular orbital calculations on lithium absorption in boron-or nitrogen-substituted disordered carbon. Carbon 2000, 38, 65–75. [Google Scholar] [CrossRef]
- Nie, M.; Lucht, B.L. Role of lithium salt on solid electrolyte interface (SEI) formation and structure in lithium-ion batteries. J. Electrochem. Soc. 2014, 161, A1001. [Google Scholar] [CrossRef]
- Arai, J.; Matsuo, A.; Fujisaki, T.; Ozawa, K. A novel high temperature stable lithium salt (Li2B12F12) for lithium-ion batteries. J. Power Sources 2009, 193, 851–854. [Google Scholar] [CrossRef]
- Yeo, J.S.; Park, T.H.; Seo, M.H.; Miyawaki, J.; Mochida, I.; Yoon, S.H. Enhancement of the rate capability of graphite via the introduction of boron-oxygen functional groups. Int. J. Electrochem. Sci. 2013, 8, 1308–1315. [Google Scholar]
- Park, M.-S.; Lee, J.; Lee, J.-W.; Kim, K.-J.; Jo, Y.-N.; Woo, S.-G.; Kim, Y.-J. Tuning the surface chemistry of natural graphite anode by H3PO4 and H3BO3 treatments for improving electrochemical and thermal properties. Carbon 2013, 62, 278–287. [Google Scholar] [CrossRef]
- Fu, L.J.; Liu, H.; Li, C.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Surface modifications of electrode materials for lithium-ion batteries. Solid State Sci. 2006, 8, 113–128. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Enhanced performance of natural graphite in Li-ion battery by oxalatoborate coating. J. Power Sources 2004, 129, 275–279. [Google Scholar] [CrossRef]
- Hwang, J.U.; Ahn, W.J.; Im, J.S.; Lee, J.D. Properties of synthetic graphite from boric acid-added pitch: Performance as anode in lithium-ion batteries. SN Appl. Sci. 2021, 3, 600. [Google Scholar] [CrossRef]
- Way, B.M.; Dahn, J.R. The Effect of Boron Substitution in Carbon on the Intercalation of Lithium in Lix(BzC1−z) 6. J. Electrochem. Soc. 1994, 141, 907–912. [Google Scholar] [CrossRef]
- Liu, T.; Luo, R.; Yoon, S.H.; Mochida, I. Anode performance of boron-doped graphites prepared from shot and sponge cokes. J. Power Sources 2010, 195, 1714–1719. [Google Scholar] [CrossRef]
- Fujimoto, H.; Mabuchi, A.; Natarajan, C.; Kasuh, T. Properties of graphite prepared from boron-doped pitch as an anode for a rechargeable Li ion battery. Carbon 2002, 40, 567–574. [Google Scholar] [CrossRef]
- Markey, B.; Zhang, M.; Robb, I.; Xu, P.; Gao, H.; Zhang, D.; Holoubek, J.; Xia, D.; Zhao, Y.; Guo, J.; et al. Effective Upcycling of Graphite Anode: Healing and Doping Enabled Direct Regeneration. J. Electrochem. Soc. 2020, 167, 160511. [Google Scholar] [CrossRef]
- Gaines, L.; Dai, Q.; Vaughey, J.T.; Gillard, S. Direct recycling R&D at the ReCell center. Recycling 2021, 6, 31. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef]
- Luo, R.-P.; Lyu, W.-Q.; Wen, K.-C.; He, W.-D. Overview of graphene as anode in lithium-ion batteries. J. Electron. Sci. Technol. 2018, 16, 57–68. [Google Scholar]
- Sui, D.; Si, L.; Li, C.; Yang, Y.; Zhang, Y.; Yan, W. A Comprehensive Review of Graphene-Based Anode Materials for Lithium-ion Capacitors. Chemistry 2021, 3, 1215–1246. [Google Scholar] [CrossRef]
- Ayala, P.; Reppert, J.; Grobosch, M.; Knupfer, M.; Pichler, T.; Rao, A.M. Evidence for substitutional boron in doped single-walled carbon nanotubes. Appl. Phys. Lett. 2010, 96, 183110. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Xu, L.; Li, F.; Cheng, H.-M. Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 2011, 5, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.; Sreena, K.P.; Vinayan, B.P.; Ramaprabhu, S. Green synthesis of boron doped graphene and its application as high performance anode material in Li ion battery. Mater. Res. Bull. 2015, 61, 383–390. [Google Scholar] [CrossRef]
- Tan, M.; Zhang, W.; Fan, C.; Li, L.; Chen, H.; Li, R.; Luo, T.; Han, S. Boric Acid–Catalyzed Hard Carbon Microfiber Derived from Cotton as a High-Performance Anode for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1801164. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Cao, Z.; Zheng, X.; Huang, W.; Wang, Y.; Qu, Q.; Zheng, H. Dynamic bonded supramolecular binder enables high-performance silicon anodes in lithium-ion batteries. J. Power Sources 2020, 463, 228208. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Moon, J.-W.; Lee, J.-G.; Baek, Y.-K.; Hong, S.-H. Porous carbon-coated silica macroparticles as anode materials for lithium ion batteries: Effect of boric acid. J. Power Sources 2014, 272, 689–695. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Recent Progress of TiO2-Based Anodes for Li Ion Batteries. J. Nanomater. 2016, 2016, 8123652. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.-H.; Jung, D.-W.; Shin, E.W.; Oh, E.-S. Boron-doped TiO2 anode materials for high-rate lithium ion batteries. J. Alloys Compd. 2014, 604, 226–232. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 spinel anode: Fundamentals and advances in rechargeable batteries. InfoMat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Sha, Y.; Zhang, Z.; Chen, Y.; Lin, Q.; Zhong, Y.; Xu, X.; Shao, Z. One-pot combustion synthesis of Li3VO4-Li4Ti5O12 nanocomposite as anode material of lithium-ion batteries with improved performance. Electrochim. Acta 2016, 222, 587–595. [Google Scholar] [CrossRef]
- Ergen, O. Hexagonal boron nitride incorporation to achieve high performance Li4Ti5O12 electrodes. AIP Adv. 2020, 10, 045040. [Google Scholar] [CrossRef]
- Su, X.; Huang, T.; Wang, Y.; Yu, A. Synthesis and electrochemical performance of nano-sized Li4Ti5O12 coated with boron-doped carbon. Electrochim. Acta 2016, 196, 300–308. [Google Scholar] [CrossRef]
- Tian, S.; Wang, X.; Yang, J. Enhanced lithium-storage performance of Li4Ti5O12 coated with boron-doped carbon layer for rechargeable Li-ion batteries. Solid State Ion. 2018, 324, 191–195. [Google Scholar] [CrossRef]
- Ren, B.; Li, W.; Wei, A.; Bai, X.; Zhang, L.; Liu, Z. Boron and nitrogen co-doped CNT/Li4Ti5O12 composite for the improved high-rate electrochemical performance of lithium-ion batteries. J. Alloy. Compd. 2018, 740, 784–789. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, C.; Li, Y.; Cai, R.; Cui, J.; Zheng, H.; Chen, D.; Zhang, Y.; Wu, Y.; Wang, Y. Li2O-2B2O3 coating decorated Li4Ti5O12 anode for enhanced rate capability and cycling stability in lithium-ion batteries. J. Colloid Interface Sci. 2021, 585, 574–582. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [Green Version]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Recent advances and remaining challenges for lithium ion battery cathodes. J. Electrochem. Soc. 2016, 164, A6220. [Google Scholar] [CrossRef]
- Nishi, Y. The development of lithium ion secondary batteries. Chem. Rec. 2001, 1, 406–413. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.J.; Kim, T.-J.; Park, B. Zero-Strain Intercalation Cathode for Rechargeable Li-Ion Cell. Angew. Chem. 2001, 113, 3471–3473. [Google Scholar] [CrossRef]
- Mauger, A.; Zhang, X.; Groult, H.; Julien, C.M. Boron Doped LiCoO2 as Cathode Materials for Rechargeable Lithium Batteries. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2011; p. 583. [Google Scholar]
- Ramkumara, B.; So-Younga, K.; Chan-Wooa, N.; Aravindanb, V.; Sunga, L.Y. LiBO2-modified LiCoO2 as an efficient cathode with garnet framework Li6.75La3Zr1.75Nb0.25O12 electrolyte toward building all-solid-state lithium battery for high-temperature operation. Electrochim. Acta 2020, 359, 136955. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, W.; Liu, Q.; Wang, Y.; Yao, X.; Qing, F.; Li, E.; Yang, T.; Zhang, L.; Li, J. Stable, fast and high-energy-density LiCoO2 cathode at high operation voltage enabled by glassy B2O3 modification. J. Power Sources 2017, 362, 131–139. [Google Scholar] [CrossRef]
- Pu, W.; Meng, Y.; Wang, Y.; Ge, Y.; Li, X.; Wang, P.; Zhang, Z.; Guo, Y.; Xiao, D. Investigation of the LiBH4 Modification Effect on Cycling Stability and High-Rate Capacity of LiCoO2 Cathodes. ACS Appl. Energy Mater. 2021, 4, 6933–6941. [Google Scholar] [CrossRef]
- Deng, T.; Fan, X.; Cao, L.; Chen, J.; Hou, S.; Ji, X.; Chen, L.; Li, S.; Zhou, X.; Hu, E.; et al. Designing in-situ-formed interphases enables highly reversible cobalt-free LiNiO2 cathode for Li-ion and Li-metal batteries. Joule 2019, 3, 2550–2564. [Google Scholar] [CrossRef]
- Eriksson, T. LiMn2O4 as a Li-Ion Battery Cathode. From Bulk to Electrolyte Interface. Ph.D. Thesis, Acta Universitatis Upsaliensis, Uppsala, Sweden, 2001. [Google Scholar]
- Liu, Q.; Zhong, L.; Guo, Y.; Xiang, M.; Su, C.; Ning, P.; Guo, J. Facile flameless combustion synthesis of high-performance boron-doped LiMn2O4 cathode with a truncated octahedra. J. Alloys Compd. 2021, 874, 159912. [Google Scholar] [CrossRef]
- Chan, H.-W.; Duh, J.-G.; Sheen, S.-R. Electrochemical performance of LBO-coated spinel lithium manganese oxide as cathode material for Li-ion battery. Surf. Coatings Technol. 2004, 188–189, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Kim, J.H.; Na Ko, Y.; Hong, Y.J.; Kang, Y.C. Electrochemical properties of Li2O–2B2O3 glass-modified LiMn2O4 powders prepared by spray pyrolysis process. J. Power Sources 2012, 210, 110–115. [Google Scholar] [CrossRef]
- Chan, H.W.; Duh, J.G.; Sheen, S.R. Surface treatment of the lithium boron oxide coated LiMn2O4 cathode material in Li-ion battery. Key Eng. Mater. 2005, 280, 671–676. [Google Scholar]
- Choi, S.H.; Kim, J.H.; Ko, Y.N.; Kang, Y.C. Electrochemical properties of boron-doped LiMn2O4 nanoparticles covered with glass material prepared by high-temperature flame spray pyrolysis. Int. J. Electrochem. Sci. 2013, 8, 1146–1162. [Google Scholar]
- Şahan, H.; Göktepe, H.; Patat, Ş.; Ülgen, A. The effect of LBO coating method on electrochemical performance of LiMn2O4 cathode material. Solid State Ion. 2008, 178, 1837–1842. [Google Scholar] [CrossRef]
- Eddrief, M.; Dzwonkowski, P.; Julien, C.; Balkanski, M. The ac conductivity in B2O3-Li2O films. Solid State Ion. 1991, 45, 77–82. [Google Scholar] [CrossRef]
- Soppe, W.; Aldenkamp, F.; Den Hartog, H.W. The structure and conductivity of binary and ternary glasses (B2O3)1−xy(Li2O)x(Li2Cl2)y. J. Non-Cryst. Solids 1987, 91, 351–374. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.-F. Past and Present of LiFePO4: From Fundamental Research to Industrial Applications. Chem 2019, 5, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Ren, Y.; Li, N. LiFePO4 cathode material. In Electric Vehicles—The Benefits and Barriers; BoD: Norderstedt, Germany, 2011; pp. 199–216. [Google Scholar]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Prosini, P.P. Determination of the Diffusion Coefficient of LiFePO4. In Iron Phosphate Materials as Cathodes for Lithium Batteries; Springer: London, UK, 2011; pp. 21–27. [Google Scholar]
- Wang, F.; Zhang, Y.; Chen, C. Research on high rate capabilities B-substituted LiFePO4. J. Nanosci. Nanotechnol. 2013, 13, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Trócoli, R.; Franger, S.; Cruz, M.; Morales, J.; Santos-Peña, J. Improving the electrochemical properties of nanosized LiFePO4-based electrode by boron doping. Electrochim. Acta 2014, 135, 558–567. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Y. High-rate and ultralong cycle-life LiFePO4 nanocrystals coated by boron-doped carbon as positive electrode for lithium-ion batteries. Appl. Surf. Sci. 2016, 390, 481–488. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, N.; Liu, Y.; Wang, J.; Yu, F.; Gu, J.; Li, W. Boron and Nitrogen Codoped Carbon Layers of LiFePO4 Improve the High-Rate Electrochemical Performance for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 20134–20143. [Google Scholar] [CrossRef] [PubMed]
- Tuo, K.; Mao, L.; Ding, H.; Dong, H.; Zhang, N.; Fu, X.; Huang, J.; Liang, W.; Li, S.; Li, C. Boron and Phosphorus Dual-Doped Carbon Coating Improves Electrochemical Performances of LiFe0.8Mn0.2PO4 Cathode Materials. ACS Appl. Energy Mater. 2021, 4, 8003–8015. [Google Scholar] [CrossRef]
- Mo, W.; Wang, Z.; Wang, J.; Li, X.; Guo, H.; Peng, W.; Yan, G. Tuning the surface of LiNi0.8Co0.1Mn0.1O2 primary particle with lithium boron oxide toward stable cycling. Chem. Eng. J. 2020, 400, 125820. [Google Scholar] [CrossRef]
- Wu, E.A.; Jo, C.; Tan, D.H.S.; Zhang, M.; Doux, J.-M.; Chen, Y.-T.; Deysher, G.; Meng, Y.S. A facile, dry-processed lithium borate-based cathode coating for improved all-solid-state battery performance. J. Electrochem. Soc. 2020, 167, 130516. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Bao, Q.; Tsai, Y.-T.; Duh, J.-G. Tuning (003) interplanar space by boric acid co-sintering to enhance Li+ storage and transfer in Li(Ni0.8Co0.1Mn0.1)O2 cathode. J. Alloys Compd. 2021, 865, 158806. [Google Scholar] [CrossRef]
- Roitzheim, C.; Kuo, L.-Y.; Sohn, Y.J.; Finsterbusch, M.; Möller, S.; Sebold, D.; Valencia, H.; Meledina, M.; Mayer, J.; Breuer, U.; et al. Boron in Ni-Rich NCM811 Cathode Material: Impact on Atomic and Microscale Properties. ACS Appl. Energy Mater. 2021, 5, 524–538. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Qiao, Y. Boosting the electrochemical performance of LiNi0.6Mn0.2Co0.2O2 through a trace amount of Mg-B co-doping. J. Mater. Sci. Technol. 2021, 89, 167–178. [Google Scholar] [CrossRef]
- Park, K.-J.; Jung, H.-G.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li-ion batteries. Adv. Energy Mater. 2018, 8, 1801202. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Luo, B.; Zhao, Z.; Shen, J.; Liu, Z.; Xiao, Z.; Zhang, B.; Zhang, J.; Ming, L.; et al. Understanding the enhancement effect of boron doping on the electrochemical performance of single-crystalline Ni-rich cathode materials. J. Colloid Interface Sci. 2021, 604, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Park, H.W.; Lee, J.D. Electrochemical Properties of Boron-doped Cathode Materials (LiNi0.90Co0.05Ti0.05O2) for Lithium-ion Batteries. Korean Chem. Eng. Res. 2019, 57, 832–840. [Google Scholar]
- Xie, Q.; Li, W.; Dolocan, A.; Manthiram, A. Insights into Boron-Based Polyanion-Tuned High-Nickel Cathodes for High-Energy-Density Lithium-Ion Batteries. Chem. Mater. 2019, 31, 8886–8897. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, G.; Zhou, K.; Zhang, J.; Jiao, T.; Zhang, X.; Yang, Y.; Zheng, J. Enhanced Interfacial Stability of a LiNi0.9Co0.05Mn0.05O2 Cathode by a Diboron Additive. ACS Appl. Energy Mater. 2021, 4, 11051–11061. [Google Scholar] [CrossRef]
- Xie, Y.; Jin, Y.; Xiang, L. Li-rich layered oxides: Structure, capacity and voltage fading mechanisms and solving strategies. Particuology 2021, 61, 1–10. [Google Scholar] [CrossRef]
- Uzun, D. Boron-doped Li1.2Mn0.6Ni0.2O2 as a cathode active material for lithium ion battery. Solid State Ionics 2015, 281, 73–81. [Google Scholar] [CrossRef]
- Pan, L.; Xia, Y.; Qiu, B.; Zhao, H.; Guo, H.; Jia, K.; Gu, Q.; Liu, Z. Structure and electrochemistry of B doped Li (Li0.2Ni0.13Co0.13Mn0.54)1−xBxO2 as cathode materials for lithium-ion batteries. J. Power Sources 2016, 327, 273–280. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, L.; Dong, C.; Zhang, H.; Zhang, M.; Liu, Y.; Zhou, Y.; Han, Y.; Chen, Y. Enhanced cycling stability of boron-doped lithium-rich layered oxide cathode materials by suppressing transition metal migration. J. Mater. Chem. A 2019, 7, 3375–3383. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. NCA, NCM811, and the Route to Ni-Richer Lithium-Ion Batteries. Energies 2020, 13, 6363. [Google Scholar] [CrossRef]
- Guilmard, M.; Croguennec, L.; Denux, D.; Delmas, C. Thermal Stability of Lithium Nickel Oxide Derivatives. Part I: LixNi1.02O2 and LixNi0.89Al0.16O2 (x = 0.50 and 0.30). Chem. Mater. 2003, 15, 4476–4483. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Park, N.-Y.; Seo, J.H.; Yu, Y.-S.; Sharma, M.; Mücke, R.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. A highly stabilized Ni-rich NCA cathode for high-energy lithium-ion batteries. Mater. Today 2020, 36, 73–82. [Google Scholar] [CrossRef]
- Chen, T.; Li, X.; Wang, H.; Yan, X.; Wang, L.; Deng, B.; Ge, W.; Qu, M. The effect of gradient boracic polyanion-doping on structure, morphology, and cycling performance of Ni-rich LiNi0.8Co0.15Al0.05O2 cathode material. J. Power Sources 2018, 374, 1–11. [Google Scholar] [CrossRef]
- Yang, W.; Xiang, W.; Chen, Y.-X.; Wu, Z.-G.; Hua, W.-B.; Qiu, L.; He, F.-R.; Zhang, J.; Zhong, B.-H.; Guo, X.-D. Interfacial regulation of Ni-rich cathode materials with an ion-conductive and pillaring layer by infusing gradient boron for improved cycle stability. ACS Appl. Mater. Interfaces 2020, 12, 10240–10251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, B.; Cheng, L.; Liu, Z.; Liu, Y.; Su, S.; Ming, L.; Zhang, J.; Ou, X. Enhanced electrochemical and structural stability of Ni-Rich cathode materials by lithium metaborate coating for lithium-ion batteries. ChemElectroChem 2022, 9, e202101395. [Google Scholar] [CrossRef]
- Yan, W.; Jia, X.; Yang, S.; Huang, Y.; Yang, P.Y.; Yuan, P.G. Synthesis of Single Crystal LiNi0.92Co0.06Mn0.01Al0.01O2 Cathode Materials with Superior Electrochemical Performance for Lithium Ion Batteries. J. Electrochem. Soc. 2020, 167, 120514. [Google Scholar] [CrossRef]
- Su, Y.; Li, L.; Chen, G.; Chen, L.; Li, N.; Lu, Y.; Bao, L.; Chen, S.; Wu, F. Strategies of Removing Residual Lithium Compounds on the Surface of Ni-Rich Cathode Materials. Chin. J. Chem. 2021, 39, 189–198. [Google Scholar] [CrossRef]
- You, L.; Chu, B.; Li, G.; Huang, T.; Yu, A. H3BO3 washed LiNi0.8Co0.1Mn0.1O2 with enhanced electrochemical performance and storage characteristics. J. Power Sources 2021, 482, 228940. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.; He, J.; Li, Y.; Wang, Y.; Liu, L.; Huang, J.; Li, C.; Wang, D.; Duan, J.; et al. Removing lithium residues via H3BO3 washing and concurrent in-situ formation of a lithium reactive coating on Ni-rich cathode materials toward enhanced electrochemical performance. Electrochim. Acta 2022, 406, 139879. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G.; Chen, L.; Li, L.; Li, C.; Ding, R.; Liu, J.; Lv, Z.; Lu, Y.; Bao, L.; et al. Clean the Ni-rich cathode material surface with boric acid to improve its storage performance. Front. Chem. 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yuen, A.C.Y.; Wang, W.; De Cachinho Cordeiro, I.M.; Wang, C.; Chen, T.B.Y.; Zhang, J.; Chan, Q.N.; Yeoh, G.H. A review on lithium-ion battery separators towards enhanced safety performances and modelling approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yanilmaz, M.; Toprakçi, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- De Moraes, A.C.M.; Hyun, W.J.; Luu, N.S.; Lim, L.-M.; Park, K.-Y.; Hersam, M.C. Phase-inversion polymer composite separators based on hexagonal boron nitride nanosheets for high-temperature lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 8107–8114. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mateti, S.; Cai, Q.; Sultana, I.; Fan, Y.; Wang, X.; Hou, C.; Chen, Y. High temperature and high rate lithium-ion batteries with boron nitride nanotubes coated polypropylene separators. Energy Storage Mater. 2019, 19, 352–359. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, Q.; Liu, M.; Han, Z.; Li, C.; Sun, C.; Chen, B.; Zhong, X.; Qiu, L.; Zhou, G. Stabilized Solid Electrolyte Interphase Induced by Ultrathin Boron Nitride Membranes for Safe Lithium Metal Batteries. Nano Lett. 2021, 21, 8447–8454. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.R.; Kim, P.J.; Kim, K.; Qi, Z.; Wang, H.; Pol, V.G. Engineered heat dissipation and current distribution boron nitride-graphene layer coated on polypropylene separator for high performance lithium metal battery. J. Colloid Interface Sci. 2021, 583, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Oh, J.; Lee, H. Review on battery thermal management system for electric vehicles. J. Appl. Therm. Eng. 2019, 149, 192–212. [Google Scholar] [CrossRef]
- Pesaran, A.A. Battery thermal management in EV and HEVs: Issues and solutions. Battery Man 2001, 43, 34–49. [Google Scholar]
- Ge, X.; Chen, Y.; Liu, W.; Zhang, G.; Li, X.; Ge, J.; Li, C. Liquid cooling system for battery modules with boron nitride based thermal conductivity silicone grease. RSC Adv. 2022, 12, 4311–4321. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Cao, M.; Du, G.; Liu, Z.; Li, W. Preparation of Boron Nitride and Silicone Rubber Composite Material for Application in Lithium Batteries. Energies 2021, 14, 999. [Google Scholar] [CrossRef]
- Meng, Z.; Dai, Z.; Chen, K.; Wang, S. Investigation on preparation, thermal, and mechanical properties of carbon fiber decorated with hexagonal boron nitride/silicone rubber composites for battery thermal management. Int. J. Energy Res. 2021, 45, 4396–4409. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Deng, J.; Zhang, G.; Zhong, Z.; He, F. Evaluation of lithium battery thermal management using sealant made of boron nitride and silicone. J. Power Sources 2020, 451, 227820. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, K.; Zhang, B.; Tong, Z.; Mao, S.; Bai, H.; Lu, Y. Ultrafast battery heat dissipation enabled by highly ordered and interconnected hexagonal boron nitride thermal conductive composites. Green Energy Environ. 2022, 7, 1401–1410. [Google Scholar] [CrossRef]
- Saw, L.H.; Ye, Y.; Tay, A.A.O. Feasibility study of Boron Nitride coating on Lithium-ion battery casing. Appl. Therm. Eng. 2014, 73, 154–161. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. New Approaches for High Energy Density Lithium–Sulfur Battery Cathodes. Accounts Chem. Res. 2012, 46, 1135–1143. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium–sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Xu, W.; Qin, S.; Das, S.; Jin, T.; Li, A.; Li, A.C.; Qie, B.; Yao, P.; Zhai, H.; et al. Full Dissolution of the Whole Lithium Sulfide Family (Li2S8 to Li2S) in a Safe Eutectic Solvent for Rechargeable Lithium–Sulfur Batteries. Angew. Chem. 2019, 131, 5613–5617. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, J.; Wu, X.; Ma, G.; Yang, J.; Wen, Z. A shuttle effect free lithium sulfur battery based on a hybrid electrolyte. Phys. Chem. Chem. Phys. 2014, 16, 21225–21229. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, X.; Liu, Y.; Wang, W.; Shao, Z. Recent progress in metal–organic frameworks for lithium–sulfur batteries. Polyhedron 2018, 155, 464–484. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kang, H.-J.; Lim, H.; Hwang, H.J.; Park, J.-W.; Lee, T.-G.; Cho, S.Y.; Jang, S.G.; Jun, Y.-S. Boron Nitride Nanotube-Based Separator for High-Performance Lithium-Sulfur Batteries. Nanomaterials 2021, 12, 11. [Google Scholar] [CrossRef]
- Babu, G.; Sawas, A.; Thangavel, N.K.; Arava, L.M.R. Two-dimensional material-reinforced separator for Li–sulfur battery. J. Phys. Chem. C 2018, 122, 10765–10772. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, D.; Rahman, M.; Tao, T.; Lei, W.; Mateti, S.; Yu, B.; Wang, J.; Yang, C.; Chen, Y. Repelling Polysulfide Ions by Boron Nitride Nanosheet Coated Separators in Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2019, 2, 2620–2628. [Google Scholar] [CrossRef]
- Han, P.; Manthiram, A. Boron- and nitrogen-doped reduced graphene oxide coated separators for high-performance Li-S batteries. J. Power Sources 2017, 369, 87–94. [Google Scholar] [CrossRef]
- Li, B.; Sun, Z.; Zhao, Y.; Tian, Y.; Tan, T.; Gao, F.; Li, J. Functional separator for Li/S batteries based on boron-doped graphene and activated carbon. J. Nanoparticle Res. 2018, 21, 7. [Google Scholar] [CrossRef]
- Eroglu, O.; Kiai, M.S.; Kizil, H. Glass fiber separator coated by boron doped anatase TiO2 for high-rate Li–S battery. Mater. Res. Bull. 2020, 129, 110917. [Google Scholar] [CrossRef]
- Luo, L.; Chung, S.; Asl, H.Y.; Manthiram, A. Long-Life Lithium–Sulfur Batteries with a Bifunctional Cathode Substrate Configured with Boron Carbide Nanowires. Adv. Mater. 2018, 30, e1804149. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Gao, Z.; Zhang, Y.; Li, X. B4C nanoskeleton enabled, flexible lithium-sulfur batteries. Nano Energy 2019, 58, 30–39. [Google Scholar] [CrossRef]
- Zhang, R.; Chi, C.; Wu, M.; Liu, K.; Zhao, T. A long-life Li–S battery enabled by a cathode made of well-distributed B4C nanoparticles decorated activated cotton fibers. J. Power Sources 2020, 451, 227751. [Google Scholar] [CrossRef]
- Guo, Y.J.; Wang, P.F.; Niu, Y.B.; Zhang, X.D.; Li, Q.; Yu, X.; Fan, M.; Chen, W.-P.; Yu, Y.; Liu, X.; et al. Boron-doped sodium layered oxide for reversible oxygen redox reaction in Na-ion battery cathodes. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carter, T.J.; Mohtadi, R.; Arthur, T.S.; Mizuno, F.; Zhang, R.; Shirai, S.; Kampf, J.W. Boron clusters as highly stable magnesium-battery electrolytes. Angew. Chem. 2014, 126, 3237–3241. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, T.; Duquesnoy, M.; El-Bouysidy, H.; Årén, F.; Gallo-Bueno, A.; Jørgensen, P.B.; Bhowmik, A.; Demortiére, A.; Ayerbe, E.; Alcaide, F.; et al. Artificial intelligence applied to battery research: Hype or reality? Chem. Rev. 2021, 122, 10899–10969. [Google Scholar] [CrossRef]








| Parameters | Requirement |
|---|---|
| Chemical and Electrochemical Stability | Yes. Long Duration |
| Wettability | Quick and Complete Wetting |
| Mechanical Property | >100 kg/cm |
| Thickness | 20–25 μm |
| Permeability | <0.025 s/μm |
| Porosity | 40–60% |
| Pore Size | <1 μm |
| Dimensional Stability | Yes |
| Thermal Stability | <5% Shrinkage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C. Examining the Benefits of Using Boron Compounds in Lithium Batteries: A Comprehensive Review of Literature. Batteries 2022, 8, 187. https://doi.org/10.3390/batteries8100187
Zheng C. Examining the Benefits of Using Boron Compounds in Lithium Batteries: A Comprehensive Review of Literature. Batteries. 2022; 8(10):187. https://doi.org/10.3390/batteries8100187
Chicago/Turabian StyleZheng, Changlin (Allen). 2022. "Examining the Benefits of Using Boron Compounds in Lithium Batteries: A Comprehensive Review of Literature" Batteries 8, no. 10: 187. https://doi.org/10.3390/batteries8100187
APA StyleZheng, C. (2022). Examining the Benefits of Using Boron Compounds in Lithium Batteries: A Comprehensive Review of Literature. Batteries, 8(10), 187. https://doi.org/10.3390/batteries8100187