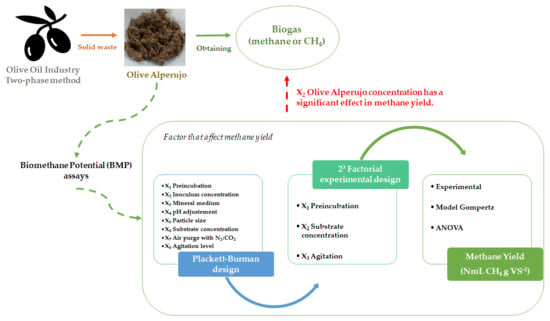
Factors That Affect Methane Yield Using Raw Olive Alperujo (Unhydrolyzed) as Substrate in BMP Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Alperujo (Substrate) and Inoculum
2.2. BMP Assays
2.3. Calculations for BMP
2.4. Statistical Analysis
2.5. Analytical Methods
3. Results
3.1. Preliminary Exploration: Plackett–Burman Design
3.2. Effect of Preincubation Time, Substrate Concentration, and Agitation Level on Methane Yield: 23 Factorial Experimental Design
4. Discussion
4.1. Raw Olive Alperujo Composition
4.2. Relevant Factors When Using Raw Olive Alperujo as Substrate in BMP Assays
4.3. Effects Preincubation Time, Substrate Concentration, and Agitation Level in Methane Yield by BMP Assays
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ChileOliva. Informe Anual Mercado Nacional de Aceite de Oliva. 2019. Available online: https://www.chileoliva.cl/wp-content/uploads/2017/04/informe-anual-mercado-nacional-de-aceite-de-oliva-2019.pdf (accessed on 15 January 2022).
- Fernández-Prior, Á.; Trujillo-Reyes, Á.; Serrano, A.; Rodríguez-Gutiérrez, G.; Reinhard, C.; Fermoso, F.G. Biogas Potential of the Side Streams Obtained in a Novel Phenolic Extraction System from Olive Mill Solid Waste. Molecules 2020, 25, 5438. [Google Scholar] [CrossRef] [PubMed]
- Hernández, D.; Astudillo, L.; Gutiérrez, M.; Tenreiro, C.; Retamal, C.; Rojas, C. Biodiesel production from an industrial residue: Alperujo. Ind. Crops Prod. 2014, 52, 495–498. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Rincón-Llorente, B.; De la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Borja-Padilla, R. Table olive wastewater: Problem, treatments and future strategy. A review. Front. Microbiol. 2018, 9, 1641. [Google Scholar] [CrossRef] [Green Version]
- Rincón, B.; Rodríguez-Gutiérrez, G.; Bujalance, L.; Fernández-Bolaños, J.; Borja, R. Influence of a steam-explosion pre-treatment on the methane yield and kinetics of anaerobic digestion of two-phase olive mil solid waste or alperujo. Process Saf. Environ. Prot. 2016, 102, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Maroto, J.M.; Uceda-Rodríguez, M.; Cobo-Ceacero, C.J.; Calero, M.; Martín-Lara, M.Á.; Cotes-Palomino, T.; López, A.B.; Martínez-García, C. Recycling of ‘alperujo’ (olive pomace) as a key component in the sintering of lightweight aggregates. J. Clean. Prod. 2019, 239, 118041. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.J.; Rincón, B.; Fermoso, F.G.; Jiménez, A.M.; Borja, R. Assessment of two-phase olive mill solid waste and microalgae co-digestion to improve methane production and process kinetics. Bioresour. Technol. 2014, 157, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Wierinck, I. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Holliger, C.; Astals, S.; Fruteau de Lacios, H.; Hafner, S.D.; Koch, K.; Weinrich, S. Towards a standardization of biomethane potential tests: A commentary. Water Sci. Technol. 2021, 83, 247–250. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, J.M.; Chynoweth, D.P. Biochemical methane potential of municipal solid waste (MSW) components. Water Sci. Technol. 1993, 27, 1–14. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Hansen, T.L.; Schmidt, J.E.; Angelidaki, I.; Marca, E.; la Cour Jansen, J.; Mosbæk, H.; Christensen, T.H. Method for determination of methane potentials of solid organic waste. Waste Manag. 2004, 24, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.; Burns, R.; Wu-Haan, W.; Spajic, R. Use of biochemical methane potential (BMP) assays for predicting and enhancing anaerobic digester performance. In Proceedings of the 44th Croatian and the 4th International Symposium on Agriculture, Opatija, Croatia, 23–26 February 2016. [Google Scholar]
- Raposo, F.; Fernández-Cegrí, V.; De la Rubia, M.A.; Borja, R.; Béline, F.; Cavinato, C.; De Wilde, V. Biochemical methane potential (BMP) of solid organic substrates: Evaluation of anaerobic biodegradability using data from an international interlaboratory study. J. Chem. Technol. Biotechnol. 2011, 86, 1088–1098. [Google Scholar] [CrossRef]
- Box, G.E.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery; Wiley-Interscience: New York, NY, USA, 2005; Volume 2. [Google Scholar]
- Montgomery, D. Diseño y análisis de Experimentos Segunda Edición; Limusa Wiley: Mexico City, Mexico, 2004. [Google Scholar]
- Soto, M.; Méndez, R.; Lema, J.M. Methanogenic and non-methanogenic activity tests. Theoretical basis and experimental set up. Water Res. 1993, 27, 1361–1376. [Google Scholar] [CrossRef]
- Hafner, S.D.; Løjborg, N.; Holliger, C.; Koch, K.; Weinrich, S. Calculation of Methane Production from Volumetric Measurements. Standard BMP Methods Document, 201, Version 1.9. 2020. Available online: https://www.dbfz.de/en/projects/bmp/methods (accessed on 25 April 2021).
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- ISO/DIS, 10707/1994; Evaluation in an Aqueous Medium of the Ultimate Aerobic Biodegradability of Organic Compounds. Method by Analysis of Biochemical Oxygen Demand (Closed Bottle Test). ISO (International Organization for Standardization): Geneva, Switzerland, 1994.
- Solarte, J.C.; Mariscal, J.P.; Aristizábal, B.H. Evaluación de la digestión y co-digestión anaerobia de residuos de comida y de poda en bioreactores a escala laboratorio. Rev. Ion 2017, 30, 105–116. [Google Scholar] [CrossRef]
- Raposo, F.; De la Rubia, M.; Fernández-Cegrí, V.; Borja, R. Anaerobic digestion of solid organic substrates in batch mode: An overview relating to methane yields and experimental procedures. Renew. Sust. Energy Rev. 2011, 16, 861–877. [Google Scholar] [CrossRef]
- Belmonte, M.; Vázquez-Padín, J.R.; Figueroa, M.; Campos, J.L.; Méndez, R.; Vidal, G.; Mosquera-Corral, A. Denitrifying activity via nitrite and N2O production using acetate and swine wastewater. Process Biochem. 2012, 47, 1202–1206. [Google Scholar] [CrossRef]
- Jamovi. 2022. Available online: https://www.jamovi.org/ (accessed on 8 October 2021).
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for Examination of Water and Wastewater, 23rd ed.; APHA: Washington, DC, USA, 2017. [Google Scholar]
- Chow, K.; Rumsey, G.; Woldroup, P. Linear programming in fish diet formulation. In Fish Feed Technology; UNDP/FAO/ADCO/REP/80/11; FAO: Rome, Italy, 1980; p. 395. [Google Scholar]
- Association of Analytical Communities (AOAC). Official Method 962.09 Fiber (Crude) in Animal Feed and Pet Food, Ceramic Fiber Filter; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- Norma Chilena (NCh) 2313/6, Of 97, Decreto Supremo Nº 317 de 1997 del Ministerio de Obras Públicas: Aguas Residuales—Métodos de análisis Parte 6: Determinación de Aceites y Grasas. Available online: https://www.inn.cl/ (accessed on 30 April 2021).
- Holliger, C.; Fruteau de Laclos, H.; Hafner, S.D.; Koch, K.; Weinrich, S.; Astals, S.; Alves, M.; Andrade, D.; Angelidaki, I.; Appels, L.; et al. Requirements for Measurement and Validation of Biochemical Methane Potential (BMP). Standard BMP Methods Document 100, Version 1.9. Available online: https://www.dbfz.de/en/BMP (accessed on 20 November 2021).
- Gallego-Fernández, L.M.; Portillo-Estévez, E.; Navarrete, B.; González, R. Estimation of methane production through the anaerobic digestion of greenhouse horticultural waste: A real case study for the Almeria region. Sci. Total Environ. 2022, 807, 151012. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Banks, C.; Siegert, I.; Heaven, S.; Borja, R. Influence of inoculum to substrate ratio on the biochemical methane potential of maize in batch tests. Process Biochem. 2006, 41, 1444–1450. [Google Scholar] [CrossRef]
- Chudoba, P.; Capdeville, B.; Chudoba, J. Explanation of biological meaning of the So/Xo ratio in batch cultivation. Water Sci. Technol. 1992, 26, 743–751. [Google Scholar] [CrossRef]
- Liu, Y. The So/Xo dependent dissolved organic carbon distribution in substrate-sufficient batch culture of activated sludge. Water Res. 2000, 34, 1645–1651. [Google Scholar] [CrossRef]
- VDI 4630; Fermentation of Organic Materials—Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. VDI—Handbuch Energietechnik: Düsseldorf, Germany, 2016.
- Vavilin, V.A.; Fernández, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef]
- Palmowski, L.M.; Müller, J.A. Influence of the size reduction of organic waste on their anaerobic digestion. Water Sci. Technol. 2000, 41, 155–162. [Google Scholar] [CrossRef]
- Sanders, W.; Geerink, M.; Zeeman, G.; Lettinga, G. Anaerobic hydrolysis kinetics of particulate substrates. Water Sci. Technol. 2000, 41, 17–24. [Google Scholar] [CrossRef]
- Mshandete, A.; Björnsson, L.; Kivaisi, A.K.; Rubindamayugi, M.S.; Mattiasson, B. Effect of particle size on biogas yield from sisal fibre waste. Renew. Energy 2006, 31, 2385–2392. [Google Scholar] [CrossRef]
- Strömberg, S.; Nistor, M.; Liu, J. Towards eliminating systematic errors caused by the experimental conditions in Biochemical Methane Potential (BMP) tests. Waste Manag. 2014, 34, 1939–1948. [Google Scholar] [CrossRef]





| Parameter | This Study | References | |||
|---|---|---|---|---|---|
| Olive Alperujo | Inoculum | [2] | [4] | [8] | |
| pH | 4.9 ± 0.1 | 7.0 ± 0.1 | 4.6 ± 0.1 | 5.3 | 4.9 ± 0.2 |
| Total solids (TS, g kg−1) | 331.8 ± 5.0 | 24.3 ± 0.0 * | 436.5 ± 0.6 ** | -- | 272.2 ± 1.7 |
| Volatile solids (VS, g kg−1) | 322.7 ± 5.2 | 15.1 ± 0.0 * | 415.8 ± 6.9 ** | -- | 234.6 ± 2.5 |
| Moisture content (%) | 74.3 ± 0.9 | -- | -- | 64.0 | 74 |
| Total nitrogen (TN, g kg−1) | 4.1 ± 0.5 | -- | -- | 11.4 | -- |
| Total protein (TP, g kg−1) | 25.4 ± 2.9 | -- | -- | 71.5 | -- |
| Total lipids (TL, g kg−1) | 45.4 ± 5.4 | -- | -- | -- | -- |
| Total fiber (TF, g kg−1) | 605.7 ± 9.9 | -- | -- | -- | -- |
| VS/TS | 1.0 | 0.6 | 1.0 | -- | 0.9 |
| Ash (g kg−1) | 43.7 *** | -- | -- | 67.4 | -- |
| Specific methanogenic activity (SMA, g COD gVSS−1·d−1) | -- | 0.6 ± 0.0 | -- | -- | -- |
| Factors | Level | Unit | ||
|---|---|---|---|---|
| −1 | +1 | |||
| X1 | Preincubation | 0 | 5 | d |
| X2 | Inoculum concentration | 2 | 26 | g VS L−1 |
| X3 | Mineral medium | No | Yes | -- |
| X4 | pH adjustment | No | Yes | -- |
| X5 | Particle size | Without milling | With milling | -- |
| X6 | Substrate concentration | 2 | 20 | g VS L−1 |
| X7 | Air purge with N2/CO2 | No | Yes | -- |
| X8 | Agitation level | 0 | 200 | rpm |
| Factors | Level | Unit | |||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| X1 | Preincubation | 0 | 3 | 6 | d |
| X2 | Substrate concentration | 2 | 11 | 20 | g VS L−1 |
| X3 | Agitation level | 0 | 100 | 200 | rpm |
| Assay | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | Methane Yield (N mL CH4 g VS−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | + | - | + | - | - | - | + | + | 158.8 ± 11.3 |
| 2 | + | - | - | - | + | + | + | - | 6.6 ± 0.6 |
| 3 | + | + | - | + | + | - | + | - | 147.5 ± 15.8 |
| 4 | - | - | - | - | - | - | - | - | 169.3 ± 0.9 |
| 5 | - | - | + | + | + | - | + | + | 231.6 ± 189.6 |
| 6 | - | + | + | + | - | + | + | - | 26.5 ± 6.2 |
| 7 | + | + | - | + | - | - | - | + | 165.7 ± 32.8 |
| 8 | - | - | - | + | + | + | - | + | 5.7 ± 1.9 |
| 9 | - | + | - | - | - | + | + | + | 36.1 ± 2.2 |
| 10 | + | - | + | + | - | + | - | - | 36.1 ± 0.6 |
| 11 | - | + | + | - | + | - | - | - | 435.1 ± 121.9 |
| 12 | + | + | + | - | + | + | - | + | 23.4 ± 6.5 |
| Assay | X1 | X2 | X3 | X1X2 | X1X3 | X2X3 | X1X2X3 | Experimental | Model | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methane Yield (N mL CH4 g VS−1) | Rm obs (N mL CH4 g VS−1·d−1) | Pm (N mL CH4 g VS−1) | Rm (N mL CH4 g VS−1·d−1) | λ (d) | ||||||||
| 1 | + | + | + | + | + | + | + | 136.0 ± 12.7 | 5.5 ± 0.4 | 338.1 | 6.4 | 7.9 |
| 2 | - | + | + | - | - | + | - | 41.7 ± 0.7 | 2.6 ± 0.0 | 42.1 | 2.6 | 1.2 |
| 3 | + | - | + | - | + | - | - | 449.3 ± 11.1 | 37.8 ± 0.8 | 433.6 | 33.0 | 3.9 |
| 4 | - | - | + | + | - | - | + | 399.0 ± 52.5 | 23.0 ± 3.0 | 403.2 | 23.6 | 1.7 |
| 5 | + | + | - | + | - | - | - | 136.8 ± 18.8 | 7.5 ± 1.1 | 269.1 | 6.6 | 8.2 |
| 6 | - | + | - | - | + | - | + | 144.1 ± 21.9 | 7.3 ± 1.0 | 461.9 | 8.0 | 11.8 |
| 7 | + | - | - | - | - | + | + | 480.2 ± 57.4 | 36.5 ± 4.4 | 509.7 | 36.7 | 8.5 |
| 8 | - | - | - | + | + | + | - | 443.8 ± 9.8 | 29.6 ± 0.6 | 476.7 | 27.6 | 6.5 |
| CP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 265.8 ± 10.6 | 32.2 ± 1.3 | 285.8 | 31.9 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, V.; Donoso-Bravo, A.; Chamy-Maggy, R.; Campos, J.L.; Mosquera-Corral, A.; Belmonte, M. Factors That Affect Methane Yield Using Raw Olive Alperujo (Unhydrolyzed) as Substrate in BMP Assays. Recycling 2022, 7, 15. https://doi.org/10.3390/recycling7020015
Ortega V, Donoso-Bravo A, Chamy-Maggy R, Campos JL, Mosquera-Corral A, Belmonte M. Factors That Affect Methane Yield Using Raw Olive Alperujo (Unhydrolyzed) as Substrate in BMP Assays. Recycling. 2022; 7(2):15. https://doi.org/10.3390/recycling7020015
Chicago/Turabian StyleOrtega, Valentina, Andrés Donoso-Bravo, Rolando Chamy-Maggy, José Luis Campos, Anuska Mosquera-Corral, and Marisol Belmonte. 2022. "Factors That Affect Methane Yield Using Raw Olive Alperujo (Unhydrolyzed) as Substrate in BMP Assays" Recycling 7, no. 2: 15. https://doi.org/10.3390/recycling7020015
APA StyleOrtega, V., Donoso-Bravo, A., Chamy-Maggy, R., Campos, J. L., Mosquera-Corral, A., & Belmonte, M. (2022). Factors That Affect Methane Yield Using Raw Olive Alperujo (Unhydrolyzed) as Substrate in BMP Assays. Recycling, 7(2), 15. https://doi.org/10.3390/recycling7020015