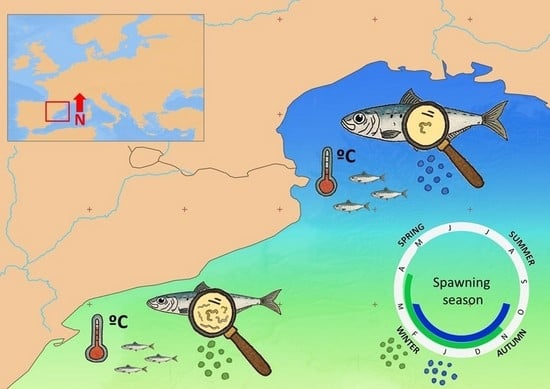
Influence of Thermal Regimes on the Relationship between Parasitic Load and Body Condition in European Sardine along the Catalan Coast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Sampling
2.2. Reproductive Cycle
2.3. Condition
2.4. Parasitological Examination
2.5. Data Analysis
3. Results
3.1. Reproductive Cycle and Energetic Condition
3.2. Characterisation of Parasitic Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cury, P.; Bakun, A.; Crawford, R.J.; Jarre, A.; Quinones, R.A.; Shannon, L.J.; Verheye, H.M. Small pelagics in upwelling systems: Patterns of interaction and structural changes in “wasp-waist” ecosystems. Ices. J. Mar. Sci. 2000, 57, 603–618. [Google Scholar] [CrossRef]
- Saraux, C.; Van Beveren, E.; Brosset, P.; Queiros, Q.; Bourdeix, J.-H.; Dutto, G.; Gasset, E.; Jac, C.; Bonhommeau, S.; Fromentin, J.-M. Small pelagic fish dynamics: A review of mechanisms in the Gulf of Lions. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 159, 52–61. [Google Scholar] [CrossRef]
- Parrish, R.H.; Serra, R.; Grant, W.S. The monotypic sardines, Sardina and Sardinops: Their taxonomy, distribution, stock structure, and zoogeography. Can. J. Fish. Aquat. 1989, 46, 2019–2036. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022; pp. 1–266. [Google Scholar]
- Sofoulaki, K.; Kalantzi, I.; Machias, A.; Pergantis, S.A.; Tsapakis, M. Metals in sardine and anchovy from Greek coastal areas: Public health risk and nutritional benefits assessment. Food Chem. Toxicol. 2019, 123, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Hamed, I.; Petričević, S.; Bogdanović, T. Seasonal Changes in Free Amino Acid and Fatty Acid Compositions of Sardines, Sardina pilchardus (Walbaum, 1792): Implications for Nutrition. Foods 2020, 9, 867. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Machias, A.; Theodorou, A. Pattern of oocyte development and batch fecundity in the Mediterranean sardine. Fish. Res. 2004, 67, 13–23. [Google Scholar] [CrossRef]
- Nunes, C.; Silva, A.; Marques, V.; Ganias, K. Integrating fish size, condition, and population demography in the estimation of Atlantic sardine annual fecundity. Cienc. Mar. 2011, 37, 565–584. [Google Scholar] [CrossRef] [Green Version]
- Albo-Puigserver, M.; Muñoz, A.; Navarro, J.; Coll, M.; Pethybridge, H.; Sánchez, S.; Palomera, I. Ecological energetics of forage fish from the Mediterranean Sea: Seasonal dynamics and interspecific differences. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 140, 74–82. [Google Scholar] [CrossRef]
- Pennino, M.G.; Coll, M.; Albo-Puigserver, M.; Fernández-Corredor, E.; Steenbeek, J.; Giráldez, A.; González, M.; Esteban, A.; Bellido, J.M. Current and future influence of environmental factors on small pelagic fish distributions in the Northwestern Mediterranean Sea. Front. Mar. Sci. 2020, 7, 622. [Google Scholar] [CrossRef]
- Caballero-Huertas, M.; Frigola-Tepe, X.; Viñas, J.; Muñoz, M. Somatic Condition and Reproductive Potential as a Tandem in European Sardine: An Analysis with an Environmental Perspective in the Northern Adriatic (Gulf of Trieste). Fishes 2022, 7, 105. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2022; pp. 1–224. [Google Scholar]
- Van Beveren, E.; Bonhommeau, S.; Fromentin, J.-M.; Bigot, J.-L.; Bourdeix, J.-H.; Brosset, P.; Roos, D.; Saraux, C. Rapid changes in growth, condition, size and age of small pelagic fish in the Mediterranean. Mar. Biol. 2014, 161, 1809–1822. [Google Scholar] [CrossRef] [Green Version]
- Brosset, P.; Lloret, J.; Muñoz, M.; Fauvel, C.; Van Beveren, E.; Marques, V.; Fromentin, J.-M.; Ménard, F.; Saraux, C. Body reserves mediate trade-offs between life-history traits: New insights from small pelagic fish reproduction. R. Soc. Open Sci. 2016, 3, 160202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quattrocchi, F.; Maynou, F. Environmental drivers of sardine (Sardina pilchardus) in the Catalan Sea (NW Mediterranean Sea). Mar. Biol. Res. 2017, 13, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, F.; Pennino, M.G.; Albo-Puigserver, M.; Steenbeek, J.; Bellido, J.M.; Coll, M. SOS small pelagics: A safe operating space for small pelagic fish in the western Mediterranean Sea. Sci. Total Environ. 2021, 756, 144002. [Google Scholar] [CrossRef]
- Brosset, P.; Fromentin, J.-M.; Van Beveren, E.; Lloret, J.; Marques, V.; Basilone, G.; Bonanno, A.; Carpi, P.; Donato, F.; Keč, V.; et al. Spatio-temporal patterns and environmental controls of small pelagic fish body condition from contrasted Mediterranean areas. Prog. Oceanogr. 2017, 151, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Ganias, K. Linking sardine spawning dynamics to environmental variability. Estuar. Coast. Shelf Sci. 2009, 84, 402–408. [Google Scholar] [CrossRef]
- Marcogliese, D.J. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev. Sci. Tech. 2008, 27, 467–484. [Google Scholar] [CrossRef]
- Diez, G.; Chust, G.; Andonegi, E.; Santurtún, M.; Abaroa, C.; Bilbao, E.; Maceira, A.; Mendibil, I. Analysis of potential drivers of spatial and temporal changes in anisakid larvae infection levels in European hake, Merluccius merluccius (L.), from the North-East Atlantic fishing grounds. Parasitol. Res. 2022, 121, 1903–1920. [Google Scholar] [CrossRef]
- Ferrer-Maza, D.; Muñoz, M.; Lloret, J.; Faliex, E.; Vila, S.; Sasal, P. Health and reproduction of red mullet, Mullus barbatus, in the western Mediterranean Sea. Hydrobiologia 2015, 753, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Maza, D.; Lloret, J.; Muñoz, M.; Faliex, E.; Vila, S.; Sasal, P. Links between parasitism, energy reserves and fecundity of European anchovy, Engraulis encrasicolus, in the northwestern Mediterranean Sea. Conserv. Physiol. 2016, 4, cov069. [Google Scholar] [CrossRef]
- Serrat, A.; Lloret, J.; Frigola-Tepe, X.; Muñoz, M. Trade-offs between life-history traits in a coldwater fish in the Mediterranean Sea: The case of blue whiting Micromesistius poutassou. J. Fish Biol. 2019, 95, 428–443. [Google Scholar] [CrossRef] [PubMed]
- Sabatés, A.; Olivar, M.P.; Salat, J.; Palomera, I.; Alemany, F. Physical and biological processes controlling the distribution of fish larvae in the NW Mediterranean. Prog. Oceanogr. 2007, 74, 355–376. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Romero, E.; Jordà, G.; Amores, A.; Kay, S.; Segura-Noguera, M.; Macias, D.M.; Maynou, F.; Sabatés, A.; Catalán, I.A. Assessment of the Skill of Coupled Physical–Biogeochemical Models in the NW Mediterranean. Front. Mar. Sci. 2020, 7, 497. [Google Scholar] [CrossRef]
- Albo-Puigserver, M.; Sánchez, S.; Coll, M.; Bernal, M.; Sáez-Liante, R.; Navarro, J.; Palomera, I. Year-round energy dynamics of sardine and anchovy in the north-western Mediterranean. Sea. Mar. Environ. 2020, 159, 105021. [Google Scholar] [CrossRef] [PubMed]
- Lloret-Lloret, E.; Albo-Puigserver, M.; Giménez, J.; Navarro, J.; Pennino, M.; Steenbeek, J.; Bellido, J.; Coll, M. Small pelagic fish fitness relates to local environmental conditions and trophic variables. Prog. Oceanogr. 2022, 202, 102745. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Machias, A.; Theodorou, A.J. Evaluation of spawning frequency in a Mediterranean sardine population (Sardina pilchardus sardina). Mar. Biol. 2003, 142, 1169–1179. [Google Scholar] [CrossRef]
- Fuentes, M.V.; Madrid, E.; Meliá, L.V.; Casañ, F.; Sáez-Durán, S.; Trelis, M.; Debenedetti, Á.L. Nematode Parasites of the European Pilchard, Sardina pilchardus (Walbaum, 1792): A Genuine Human Hazard? Animals 2022, 12, 1877. [Google Scholar] [CrossRef]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A standardized terminology for describing reproductive development in fishes. Mar. Coast. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Nikolsky, G.V. The Ecology of Fishes; Academic Press: London, UK, 1963. [Google Scholar]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef] [Green Version]
- Green, A.J. Mass/length residuals: Measures of body condition or generators of spurious results? Ecology 2001, 82, 1473–1483. [Google Scholar] [CrossRef]
- Bayse, S.M.; Regish, A.M.; McCormick, S.D. Proximate composition, lipid utilization and validation of a non-lethal method to determine lipid content in migrating American shad Alosa sapidissima. J. Fish Biol. 2018, 92, 1832–1848. [Google Scholar] [CrossRef] [PubMed]
- Kent, M. Hand-held instrument for fat/water determination in whole fish. Food Control 1990, 1, 47–53. [Google Scholar] [CrossRef]
- Schloesser, R.W.; Fabrizio, M.C. Condition indices as surrogates of energy density and lipid content in juveniles of three fish species. Trans. Am. Fish. Soc. 2017, 146, 1058–1069. [Google Scholar] [CrossRef] [Green Version]
- Brosset, P.; Fromentin, J.-M.; Ménard, F.; Pernet, F.; Bourdeix, J.-H.; Bigot, J.-L.; Van Beveren, E.; Roda, M.A.P.; Choy, S.; Saraux, C. Measurement and analysis of small pelagic fish condition: A suitable method for rapid evaluation in the field. J. Exp. Mar. Biol. Ecol. 2015, 462, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Van Der Lingen, C.D.; Hutchings, L. Estimating the lipid content of pelagic fish in the southern Benguela by visual assessment of their mesenteric fat. Afr. J. Mar. Sci. 2005, 27, 45–53. [Google Scholar] [CrossRef]
- Petter, A.J.; Maillard, C. Larves d’ascarides parasites de poissons en Méditerranée occidentale. Bull. Mus. Natl. Hist. Nat. 1988, 10, 347–369. [Google Scholar]
- Petter, A.J.; Cabaret, J.; Tcheprakoff, R. Ascaridoid nematodes of teleostean fishes from the eastern North Atlantic and seas of the north of Europe. Parasite 1995, 2, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Navone, G.T.; Sardella, N.H.; Timi, J.T. Larvae and adults of Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda: Anisakidae) in fishes and crustaceans in the South West Atlantic. Parasite 1998, 5, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábian, I.; Rózsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 1–4. [Google Scholar] [CrossRef]
- Rózsa, L.; Reiczigel, J.; Majoros, G. Quantifying parasites in samples of hosts. J. Parasitol. 2000, 86, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Mattiucci, S.; Fazii, P.; De Rosa, A.; Paoletti, M.; Megna, A.S.; Glielmo, A.; De Angelis, M.; Costa, A.; Meucci, C.; Calvaruso, V.; et al. Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii infection, Italy. Emerg. Infect. Dis. 2013, 19, 496. [Google Scholar] [CrossRef] [PubMed]
- Bušelić, I.; Botić, A.; Hrabar, J.; Stagličić, N.; Cipriani, P.; Mattiucci, S.; Mladineo, I. Geographic and host size variations as indicators of Anisakis pegreffii infection in European pilchard (Sardina pilchardus) from the Mediterranean Sea: Food safety implications. Int. J. Food Microbiol. 2018, 266, 126–132. [Google Scholar] [CrossRef]
- Biton-Porsmoguer, S.; Bou, R.; Lloret, E.; Alcaide, M.; Lloret, J. Fatty acid composition and parasitism of European sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) populations in the northern Catalan Sea in the context of changing environmental conditions. Conserv. Physiol. 2020, 8, coaa121. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Nagasawa, K.; Ishikura, H.; Nakagawa, A.; Sato, N.; Kikuchi, K.; Ishikura, H. Female worm Hysterothylacium aduncum excreted from human: A case report. Jpn. J. Parasitol. 1996, 45, 12–23. [Google Scholar]
- Shamsi, S.; Butcher, A.R. First report of human anisakidosis in Australia. Med. J. Aust. 2011, 194, 199–200. [Google Scholar] [CrossRef]
- Cavallero, S.; Magnabosco, C.; Civettini, M.; Boffo, L.; Mingarelli, G.; Buratti, P.; Giovanardi, O.; Fortuna, C.M.; Arcangeli, G. Survey of Anisakis sp. and Hysterothylacium sp. in sardines and anchovies from the North Adriatic Sea. Int. J. Food Microbiol. 2015, 200, 18–21. [Google Scholar] [CrossRef]
- Balbuena, J.A.; Karlsbakk, E.; Kvenseth, A.M.; Saksvik, M.; Nylund, A. Growth and emigration of third-stage larvae of Hysterothylacium aduncum (Nematoda: Anisakidae) in larval herring Clupea harengus. J. Parasitol. 2000, 86, 1271–1275. [Google Scholar] [CrossRef]
- Dallarés, S.; Moyà-Alcover, C.M.; Padrós, F.; Cartes, J.E.; Solé, M.; Castañeda, C.; Carrassón, M. The parasite community of Phycis blennoides (Brünnich, 1768) from the Balearic Sea in relation to diet, biochemical markers, histopathology and environmental variables. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 118, 84–100. [Google Scholar] [CrossRef]
- Chen, C.T.; Carlotti, F.; Harmelin-Vivien, M.; Guilloux, L.; Bănaru, D. Temporal variation in prey selection by adult European sardine (Sardina pilchardus) in the NW Mediterranean Sea. Prog. Oceanogr. 2021, 196, 102617. [Google Scholar] [CrossRef]
- Køie, M. Aspects of the life cycle and morphology of Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda, Ascaridoidea, Anisakidae). Can. J. Zool. 1993, 71, 1289–1296. [Google Scholar] [CrossRef]
- Costalago, D.; Palomera, I. Feeding of European pilchard (Sardina pilchardus) in the northwestern Mediterranean: From late larvae to adults. Sci. Mar. 2014, 78, 41–54. [Google Scholar] [CrossRef]
- Klimpel, S.; Rückert, S. Life cycle strategy of Hysterothylacium aduncum to become the most abundant anisakid fish nematode in the North Sea. Parasitol. Res. 2005, 97, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Rello, F.J.; Adroher, F.J.; Benítez, R.; Valero, A. The fishing area as a possible indicator of the infection by anisakids in anchovies (Engraulis encrasicolus) from southwestern Europe. Int. J. Food Microbiol. 2009, 129, 277–281. [Google Scholar] [CrossRef]
- Carvajal, J.; González, L.; Toledo, G. New record of Hysterothylacium aduncum (Rudolphi, 1802)(Nematoda: Anisakidae) in salmonids cultured in sea farms from southern Chile. Res. Rev. Parasitol. 1995, 55, 195–197. [Google Scholar]
- Bosc, E.; Bricaud, A.; Antoine, D. Seasonal and interannual variability in algal biomass and primary production in the Mediterranean Sea, as derived from 4 years of SeaWiFS observations. Global Biogeochem Cycles. 2004, 18, GB1005. [Google Scholar] [CrossRef] [Green Version]
- Estrada, M.; Latasa, M.; Emelianov, M.; Gutiérrez-Rodríguez, A.; Fernández-Castro, B.; Isern-Fontanet, J.; Mouriño-Carballido, B.; Salat, J.; Vidal, M. Seasonal and mesoscale variability of primary production in the deep winter-mixing region of the NW Mediterranean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2014, 94, 45–61. [Google Scholar] [CrossRef]
- Nunes, S.; Latasa, M.; Gasol, J.M.; Estrada, M. Seasonal and interannual variability of phytoplankton community structure in a Mediterranean coastal site. Mar. Ecol. Prog. Ser. 2018, 592, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Pethybridge, H.; Bodin, N.; Arsenault-Pernet, E.; Bourdeix, J.; Brisset, B.; Bigot, J.; Roos, D.; Peter, M. Temporal and inter-specific variations in forage fish feeding conditions in the NW Mediterranean: Lipid content and fatty acid compositional changes. Mar. Ecol. Prog. Ser. 2014, 512, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Brosset, P.; Ménard, F.; Fromentin, J.; Bonhommeau, S.; Ulses, C.; Bourdeix, J.; Bigot, J.; Van Beveren, E.; Roos, D.; Saraux, C. Influence of environmental variability and age on the body condition of small pelagic fish in the Gulf of Lions. Mar. Ecol. Prog. Ser. 2015, 529, 219–231. [Google Scholar] [CrossRef] [Green Version]
- Báez, J.C.; Pennino, M.G.; Albo-Puigserver, M.; Coll, M.; Giraldez, A.; Bellido, J.M. Effects of environmental conditions and jellyfish blooms on small pelagic fish and fisheries from the Western Mediterranean Sea. Estuar. Coast. Shelf Sci. 2022, 264, 107699. [Google Scholar] [CrossRef]
- Bellido, J.M.; Brown, A.M.; Valavanis, V.D.; Giráldez, A.; Pierce, G.J.; Iglesias, M.; Palialexis, A. Identifying essential fish habitat for small pelagic species in Spanish Mediterranean waters. In Essential Fish Habitat Mapping in the Mediterranean. Hydrobiologia 2008, 14, 171–184. [Google Scholar] [CrossRef]
- Hidalgo, M.; Vasilakopoulos, P.; García-Ruiz, C.; Esteban, A.; López-López, L.; García-Gorriz, E. Resilience dynamics and productivity-driven shifts in the marine communities of the Western Mediterranean Sea. J. Anim. Ecol. 2021, 91, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E.M.; Simó, R.; Coma, R.; Ribes, M.; Pascual, J.; Sabates, A.; Gili, J.M.; Pelejero, C. Effects of climate change on Mediterranean marine ecosystems: The case of the Catalan Sea. Clim. Res. 2011, 50, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Severin, T.; Conan, P.; de Madron, X.D.; Houpert, L.; Oliver, M.; Oriol, L.; Caparros, J.; Ghiglione, J.; Pujo-Pay, M. Impact of open-ocean convection on nutrients, phytoplankton biomass and activity. Deep Sea Res. Part I Oceanogr. Res. 2014, 94, 62–71. [Google Scholar] [CrossRef]
- Oguz, T.; Macias, D.; Tintore, J. Ageostrophic frontal processes controlling phytoplankton production in the Catalano-Balearic Sea (Western Mediterranean). PLoS ONE 2015, 10, e0129045. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Corredor, E.; Albo-Puigserver, M.; Pennino, M.G.; Bellido, J.M.; Coll, M. Influence of environmental factors on different life stages of European anchovy (Engraulis encrasicolus) and European sardine (Sardina pilchardus) from the Mediterranean Sea: A literature review. Reg. Stud. Mar. Sci. 2021, 41, 101606. [Google Scholar] [CrossRef]
- Salat, J.; Garcia, M.A.; Cruzado, A.; Palanques, A.; Arín, L.; Gomis, D.; Guillén, J.; de León, A.; Puigdefàbregas, J.; Sospedra, J.; et al. Seasonal changes of water mass structure and shelf slope exchanges at the Ebro Shelf (NW Mediterranean). Cont Shelf Res. 2002, 22, 327–348. [Google Scholar] [CrossRef]
- Garrido, S.; Ben-Hamadou, R.; Oliveira, P.B.; Cunha, M.E.; Chícharo, M.A.; van der Lingen, C.D. Diet and feeding intensity of sardine Sardina pilchardus: Correlation with satellite-derived chlorophyll data. Mar. Ecol. Prog. Ser. 2008, 354, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Costalago, D.; Palomera, I.; Tirelli, V. Seasonal comparison of the diets of juvenile European anchovy Engraulis encrasicolus and sardine Sardina pilchardus in the Gulf of Lions. J. Sea Res. 2014, 89, 64–72. [Google Scholar] [CrossRef]





| Autumn | Winter | Spring | Summer | |
|---|---|---|---|---|
| North | 224 | 241 | 149 | 233 |
| South | 175 | 181 | 80 | 92 |
| NI | Prevalence | Mean Intensity ± SD | Mean abundance ± SD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyst | CA | Total | Digenean | Hyst | CA | Total | Digenean | Hyst | CA | Total | Hyst | CA | Total | |
| North n = 847 | 276 | 4 | 278 | 106 | 32.6 | 0.5 | 32.8 | 12.5 | 1.500 ± 0.851 | 1.000 ± 0.000 | 1.504 ± 0.857 | 0.489 ± 0.854 | 0.005 ± 0.069 | 0.493 ± 0.860 |
| South n = 528 | 206 | 2 | 207 | 71 | 39.0 | 0.4 | 39.2 | 13.4 | 1.922 ± 1.433 | 1.000 ± 0.000 | 1.923 ± 1.433 | 0.750 ± 1.296 | 0.004 ± 0.061 | 0.754 ± 1.298 |
| p | - | - | - | 0.017 | 1.000 | 0.017 | 0.620 | 0.001 | - | 0.002 | 0.001 | 0.789 | 0.001 | |
| N | Ni | Prevalence (%) | Mean Intensity ± SD | Mean Abundance ± SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North | South | North | South | North | South | p-Value | North | South | p-Value | North | South | p-Value | |
| Autumn | 224 | 175 | 82 | 70 | 36.6 | 40.0 | 0.533 | 1.646 ± 0.986 | 2.129 ± 1.769 | 0.054 | 0.603 ± 0.992 | 0.851 ± 1.528 | 0.066 |
| Winter | 241 | 181 | 106 | 83 | 44.0 | 45.9 | 0.767 | 1.519 ± 0.864 | 1.928 ± 1.395 | 0.029 | 0.668 ± 0.948 | 0.884 ± 1.347 | 0.075 |
| Spring | 149 | 80 | 47 | 45 | 31.5 | 56.2 | 0.000 | 1.532 ± 0.830 | 1.756 ± 0.908 | 0.216 | 0.483 ± 0.851 | 0.988 ± 1.108 | 0.002 |
| Summer | 233 | 92 | 43 | 9 | 18.5 | 9.8 | 0.065 | 1.163 ± 0.433 | 1.111 ± 0.333 | 0.689 | 0.215 ± 0.488 | 0.109 ± 0.346 | 0.036 |
| p-value | - | - | - | - | <0.000 | <0.000 | - | 0.303 | 0.232 | - | 0.844 | 0.883 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigola-Tepe, X.; Caballero-Huertas, M.; Viñas, J.; Muñoz, M. Influence of Thermal Regimes on the Relationship between Parasitic Load and Body Condition in European Sardine along the Catalan Coast. Fishes 2022, 7, 358. https://doi.org/10.3390/fishes7060358
Frigola-Tepe X, Caballero-Huertas M, Viñas J, Muñoz M. Influence of Thermal Regimes on the Relationship between Parasitic Load and Body Condition in European Sardine along the Catalan Coast. Fishes. 2022; 7(6):358. https://doi.org/10.3390/fishes7060358
Chicago/Turabian StyleFrigola-Tepe, Xènia, Marta Caballero-Huertas, Jordi Viñas, and Marta Muñoz. 2022. "Influence of Thermal Regimes on the Relationship between Parasitic Load and Body Condition in European Sardine along the Catalan Coast" Fishes 7, no. 6: 358. https://doi.org/10.3390/fishes7060358
APA StyleFrigola-Tepe, X., Caballero-Huertas, M., Viñas, J., & Muñoz, M. (2022). Influence of Thermal Regimes on the Relationship between Parasitic Load and Body Condition in European Sardine along the Catalan Coast. Fishes, 7(6), 358. https://doi.org/10.3390/fishes7060358