Stomach Content Analysis for Juvenile Great Hammerhead Sharks Sphyrna mokarran (Rüppell, 1837) from the Arabian Gulf
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Stomach Fullness
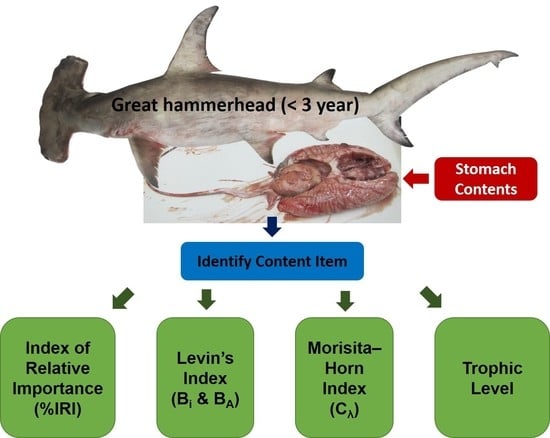
2.3. Stomach Content Analysis
2.4. Trophic Level
3. Results
3.1. Gear and Stomach Fullness
3.2. Stomach Content Analysis
3.3. Niche Breadth and Trophic Overlap
3.4. Trophic Level
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Compagno, L.J.V. Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date; FAO Fisheries Synopsis; FAO Species Catalogue; Food and Agriculture Organization of the United Nations: Rome, Italy, 1984; Volume 4, pp. 251–655. [Google Scholar]
- Cliff, G. Sharks caught in the protective gill nets off KwaZulu-Natal, South Africa. 8. The Great hammerhead shark Sphyrna mokarran (Rüppell). S. Afr. J. Mar. Sci. 1995, 15, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Ebert, D.A.; Fowler, S.; Compagno, L. Sharks of the World—A Fully Illustrated Guide; Wild Nature Press: Plymouth, UK, 2013; p. 528. [Google Scholar]
- Mourier, J.; Planes, S.; Buray, N. Trophic interactions at the top of the coral reef food chain. Coral Reefs 2013, 32, 285. [Google Scholar] [CrossRef] [Green Version]
- Guttridge, T.L.; Van Zinnicq Bergmann, M.P.M.; Bolte, C.; Howey, L.A.; Finger, J.S.; Kessel, S.T.; Brooks, J.L.; Winram, W.; Bond, M.E.; Jordan, L.K.B.; et al. Philopatry and regional connectivity of the great hammerhead shark, Sphyrna mokarran in the U.S. and the Bahamas. Front. Mar. Sci. 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, A.J.; Klimley, A.P. The biology and conservation status of the large hammerhead shark complex: The great, scalloped, and smooth hammerheads. Rev. Fish Biol. Fish. 2018, 28, 777–794. [Google Scholar] [CrossRef]
- Piercy, A.N.; Carlson, J.K.; Passerotti, M.S. Age and growth of the great hammerhead shark, Sphyrna mokarran, in the north-western Atlantic Ocean and Gulf of Mexico. Mar. Freshw. Res. 2010, 61, 992–998. [Google Scholar] [CrossRef]
- Gulak, S.J.B.; de Ron Santiago, A.J.; Carlson, J.K. Hooking mortality of scalloped hammerhead Sphyrna lewini and great hammerhead Sphyrna mokarran sharks caught on bottom longlines. Afr. J. Mar. Sci. 2015, 37, 267–273. [Google Scholar] [CrossRef]
- Jabado, R.W.; Kyne, P.M.; Pollom, R.A.; Ebert, D.A.; Simpfendorfer, C.A.; Ralph, G.M.; Dulvy, N.K. The Conservation Status of Sharks, Rays, and Chimaeras in the Arabian Sea and Adjacent Waters; Environment Agency—Abu Dhabi and IUCN Species Survival Commission Shark Specialist Group: Abu Dhabi, United Arab Emirates, 2017; p. 230. [Google Scholar]
- Checklist of CITES Species. Available online: http://checklist.cites.org/#/en (accessed on 21 June 2022).
- Appendices I and II of the Convention on the Conservation of Migratory Species of Wild Animals (CMS). Available online: https://goo.gl/Ta4agj (accessed on 21 June 2022).
- Rigby, C.L.; Barreto, R.; Carlson, J.; Fernando, D.; Fordham, S.; Francis, M.P.; Herman, K.; Jabado, R.W.; Liu, K.M.; Marshall, A.; et al. Sphyrna mokarran; The IUCN Red List of Threatened Species 2019: e.T39386A2920499; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2019; Available online: https://dx.doi.org/10.2305/IUCN.UK.2019-3.RLTS.T39386A2920499.en (accessed on 27 November 2022).
- Hsu, H.H.; Nazeer, Z.M.; Lin, Y.J.; Panickan, P.; Al-Abdulkader, K.; Loughland, R.; Qurban, M.A. Biological aspects of juvenile great hammerhead sharks Sphyrna mokarran from the Arabian Gulf. Mar. Freshw. Res. 2021, 72, 110–117. [Google Scholar] [CrossRef]
- Passerotti, M.S.; Carlson, J.K.; Piercy, A.N.; Campana, S.E. Age validation of great hammerhead shark (Sphyrna mokarran), determined by bomb radiocarbon analysis. Fish. Bull. 2010, 108, 346–351. [Google Scholar]
- Harry, A.V.; Macbeth, W.G.; Gutteridge, A.N.; Simpfendorfer, C.A. The life histories of endangered hammerhead sharks (Carcharhiniformes, Sphyrnidae) from the east coast of Australia. J. Fish Biol. 2011, 78, 2026–2051. [Google Scholar] [CrossRef]
- Tovar-Ávila, J.; Gallegos-Camacho, R. Oldest estimated age for Sphyrna mokarran (Carcharhiniformes: Sphyrnidae) in the Mexican Pacific. Hidrobiológica 2014, 24, 163–165. [Google Scholar]
- Stevens, J.D.; Lyle, J.M. Biology of three hammerhead sharks (Eusphyra blochii, Sphyrna mokarran and S. lewini) from Northern Australia. Mar. Freshw. Res. 1989, 40, 129–146. [Google Scholar] [CrossRef]
- Bass, A.J.; D’Aubrey, J.D.; Kistnasamy, N. Sharks of the east coast of Southern Africa. III. In The Families Carcharhinidae (Excluding Mustelus and Carcharhinus) and Sphyrnidae; Oceanography Research Institute: Durban, South Africa, 1975; Volume 38, pp. 44–45. [Google Scholar]
- Strong, W.R., Jr.; Snelson, F.F., Jr.; Gruber, S.H. Hammerhead shark predation on stingrays: An observation of prey handling by Sphyrna mokarran. Copeia 1990, 1990, 836–840. [Google Scholar] [CrossRef]
- Raoult, V.; Broadhurst, M.K.; Peddemors, M.; Williamson, M.E.; Gaston, M.F. Resource use of great hammerhead sharks Sphyrna mokarran off eastern Australia. J. Fish Biol. 2019, 95, 1430–1440. [Google Scholar] [CrossRef]
- Cortés, E. Standardized diet compositions and trophic levels of sharks. ICES J. Mar. Sci. 1999, 56, 707–717. [Google Scholar] [CrossRef]
- Rastgoo, A.R.; Navarro, J.; Valinassab, T. Comparative diets of sympatric batoid elasmobranchs in the Gulf of Oman. Aquat. Biol. 2018, 27, 35–41. [Google Scholar] [CrossRef]
- Cortés, E. A critical review of methods of studying fish feeding based on analysis of stomach contents: Application to elasmobranch fishes. Can. J. Fish. Aquat. Sci. 1997, 54, 726–738. [Google Scholar] [CrossRef]
- Estupiñán-Montaño, C.; Cedeño-Figueroa, L.; Estupiñán-Ortiz, J.F.; Galván-Magaña, F.; Sandoval-Londoño, A.; Castañeda-Suarez, D.; Polo-Silva, V.J. Feeding habits and trophic level of the smooth hammerhead shark, Sphyrna zygaena (Carcharhiniformes: Sphyrnidae), off Ecuador. J. Mar. Biol. Assoc. U. K. 2019, 99, 673–680. [Google Scholar] [CrossRef] [Green Version]
- D’Iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and ecological aspects of the blackmouth catshark (Galeus melastomus Rafinesque, 1810) in the southern Tyrrhenian Sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- Smith, E.P.; Zaret, T.M. Bias in estimating niche overlap. Ecology 1982, 63, 1248–1253. [Google Scholar] [CrossRef]
- Jabado, R.W.; Al-Ghais, S.M.; Hamza, W.; Shivji, M.S.; Henderson, A.C. Shark diversity in the Arabian/Persian Gulf higher than previously thought: Insights based on species composition of shark landings in the United Arab Emirates. Mar. Biodivers. 2015, 45, 719–731. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication, Version (12/2019). Available online: www.fishbase.org (accessed on 5 September 2022).
- Palomares, M.L.D.; Pauly, D. SeaLifeBase. World Wide Web Electronic Publication, Version (07/2020). 2020. Available online: www.sealifebase.org (accessed on 5 September 2022).
- Guo, K.; Zhao, W.; Wang, S.; Liu, B.; Zhang, P. Food web structure and trophic levels in a saltwater pond sea cucumber and prawn polyculture system. Acta Oceanol. Sin. 2016, 35, 58–62. [Google Scholar] [CrossRef]
- Hsu, H.H.; Yacoubi, L.; Lin, Y.-J.; Le Loc’h, F.; Katsanevakis, S.; Giovos, I.; Qurban, M.A.; Nazeer, Z.; Panickan, P.; Maneja, R.H.; et al. Elasmobranchs of the western Arabian Gulf: Diversity, status, and implications for conservation. Reg. Stud. Mar. Sci. 2022, 56, 102637. [Google Scholar] [CrossRef]
- Torres-Rojas, Y.E.; Hernandez-Herrera, A.; Galván-Magaña, F. Feeding habits of the scalloped hammerhead shark, Sphyrna lewini, in Mazatlán waters, southern Gulf of California, Mexico. Cybium 2007, 30, 85–90. [Google Scholar]
- Lai, W. Analyzes of Stomach Contents of Four Large Shark Species in the Waters of Northeastern Taiwan. Master Thesis, National Taiwan Ocean University, Keelung, Taiwan, June 2011. (In Chinese with English Abstract). [Google Scholar]
- Yeh, S.Y. Feeding Ecology of Two Hammerhead Shark Species from Northeastern Taiwan Waters. Master Thesis, National Taiwan Ocean University, Keelung, Taiwan, July 2017. (In Chinese with English Abstract). [Google Scholar]
- Galindo, E.; Giraldo, A.; Navia, A.F. Feeding habits and trophic interactions of four sympatric hammerhead shark species reveal trophic niche partitioning. Mar. Ecol. Prog. Ser. 2021, 665, 159–175. [Google Scholar] [CrossRef]
- Smale, M.J. Occurrence and feeding of three shark species, Carcharhinus brachyurus, C. obscurus and Sphyrna zygaena, on the Eastern Cape coast of South Africa. S. Afr. J. Mar. Sci. 1991, 11, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Torres-Rojas, Y.E.; Páez Osuna, F.; Camalich, J.; Galván-Magaña, F. Diet and trophic level of scalloped hammerhead shark (Sphyrna lewini) from the Gulf of California and Gulf of Tehuantepec, Mexico. Iran. J. Fish. Sci. 2015, 14, 767–785. [Google Scholar]
- D’Iglio, C.; Savoca, S.; Rinelli, P.; Spanò, N.; Capillo, G. Diet of the deep-Sea shark Galeus melastomus Rafinesque, 1810, in the Mediterranean Sea: What we know and what we should know. Sustainability 2021, 13, 3962. [Google Scholar] [CrossRef]
- Cortés, E.; Manire, C.A.; Hueter, R.E. Diet, feeding habits, and diel feeding chronology of the bonnethead shark, Sphyrna tiburo, in southwest Florida. Bull. Mar. Sci. 1996, 58, 353–367. [Google Scholar]
- Leigh, S.C.; Papastamatiou, Y.P.; German, D.P. Seagrass digestion by a notorious ‘carnivore’. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181583. [Google Scholar] [CrossRef] [Green Version]
- Hueter, R.E.; Tyminski, J.P. Species-specific distribution and habitat characteristics of shark nurseries in Gulf of Mexico waters off peninsular Florida and Texas. Am. Fish. Soc. Symp. 2007, 50, 193–223. [Google Scholar]
- Roemer, R.P.; Gallagher, A.J.; Hammerschlag, N. Shallow water tidal flat use and associated specialized foraging behavior of the great hammerhead shark (Sphyrna mokarran). Mar. Freshw. Behav. Physiol. 2016, 49, 235–249. [Google Scholar] [CrossRef]
- Barker, A.M.; Frazier, B.S.; Bethea, D.M.; Gold, J.R.; Portnoy, D.S. Identification of young-of-the-year great hammerhead shark Sphyrna mokarran in northern Florida and South Carolina. J. Fish Biol. 2017, 91, 664–668. [Google Scholar] [CrossRef]
- Macdonald, C.; Jerome, J.; Pankow, C.; Perni, N.; Black, K.; Shiffman, D.; Wester, J. First identification of probable nursery habitat for critically endangered great hammerhead Sphyrna mokarran on the Atlantic Coast of the United States. Conserv. Sci. Pract. 2021, 3, e418. [Google Scholar] [CrossRef]
- Hammerschlag, N. Quantifying shark predation effects on prey: Dietary data limitations and study approaches. Endanger. Species Res. 2019, 38, 147–151. [Google Scholar] [CrossRef]

| Content Items | %N | %W | %O | IRI | %IRI |
|---|---|---|---|---|---|
| Cephalopods | 2.60 | 1.48 | 5.08 | 22.25 | 0.18 |
| Octopus cyaneus Gray, 1849 | 0.65 | 0.23 | 1.82 | 1.59 | 0.06 |
| Fam. Sepiidae | 1.30 | 1.24 | 1.82 | 4.62 | 0.18 |
| Cephalopod remains | 0.65 | 0.02 | 1.82 | 1.21 | 0.05 |
| Elasmobranchs | 5.19 | 21.92 | 11.86 | 345.07 | 2.84 |
| Maculabatis randalli (Last, Manjaji-Matsumoto & Moore, 2012) | 0.65 | 11.02 | 1.82 | 21.22 | 0.84 |
| Fam. Dasyatidae | 0.65 | 4.82 | 1.82 | 9.95 | 0.39 |
| Fam. Myliobatidae | 3.25 | 5.93 | 7.27 | 66.74 | 2.64 |
| Ord. Myliobatiformes | 0.65 | 0.14 | 1.82 | 1.44 | 0.06 |
| Teleosts | 54.55 | 73.32 | 83.05 | 13,948.84 | 93.81 |
| Saurida tumbil (Bloch, 1795) | 1.30 | 5.77 | 3.64 | 25.70 | 1.02 |
| Saurida spp. | 1.95 | 4.81 | 3.64 | 24.56 | 0.97 |
| Grammoplites suppositus (Troschel, 1840) | 1.95 | 4.63 | 3.64 | 23.93 | 0.95 |
| Platycephalus indicus (Linnaeus, 1758) | 4.55 | 14.81 | 10.91 | 211.15 | 8.34 |
| Epinephelus coioides (Hamilton, 1822) | 0.65 | 7.26 | 1.82 | 14.37 | 0.57 |
| Epinephelus spp. | 1.30 | 9.23 | 1.82 | 19.15 | 0.76 |
| Sillago sihama (Forsskål, 1775) | 1.30 | 0.26 | 1.82 | 2.83 | 0.11 |
| Megalaspis cordyla (Linnaeus, 1758) | 0.65 | 1.51 | 1.82 | 3.92 | 0.15 |
| Lutjanus ehrenbergii (Peters, 1869) | 0.65 | 2.47 | 1.82 | 5.68 | 0.22 |
| Gerres spp. | 0.65 | 0.24 | 1.82 | 1.61 | 0.06 |
| Acanthopagrus bifasciatus (Forsskål, 1775) | 1.30 | 1.21 | 3.64 | 9.12 | 0.36 |
| Lethrinus nebulosus (Forsskål, 1775) | 0.65 | 0.42 | 1.82 | 1.94 | 0.08 |
| Nemipterus japonicus (Bloch, 1791) | 0.65 | 1.22 | 1.82 | 3.41 | 0.13 |
| Otolithes ruber (Bloch & Schneider, 1801) | 1.30 | 0.38 | 1.82 | 3.05 | 0.12 |
| Siganus canaliculatus (Park, 1797) | 0.65 | 4.71 | 1.82 | 9.75 | 0.39 |
| Pseudorhombus spp. | 1.30 | 0.66 | 1.82 | 3.56 | 0.14 |
| Fam. Platycephalidae | 3.90 | 4.14 | 10.91 | 87.69 | 3.46 |
| Fam. Serranidae | 0.65 | 1.98 | 1.82 | 4.78 | 0.19 |
| Fam. Scombridae | 0.65 | 0.57 | 1.82 | 2.22 | 0.09 |
| Fish remains | 28.57 | 7.04 | 49.09 | 1748.29 | 69.06 |
| Crustaceans | 5.84 | 3.10 | 11.86 | 113.80 | 0.94 |
| Marsupenaeus japonicus (Spence Bate, 1888) | 0.65 | 1.13 | 1.82 | 3.24 | 0.13 |
| Penaeus semisulcatus De Haan, 1844 | 1.30 | 0.57 | 1.82 | 3.39 | 0.13 |
| Fam. Penaeidae | 3.25 | 1.39 | 7.27 | 33.75 | 1.33 |
| Crustacean remains | 0.65 | 0.01 | 1.82 | 1.19 | 0.05 |
| Bivalves | 0.65 | <0.01 | 1.69 | 1.18 | 0.01 |
| Bivalve remains | 0.65 | <0.01 | 1.82 | 1.18 | 0.05 |
| Plants | 6.49 | 0.01 | 1.69 | 23.64 | 0.10 |
| Sargassum angustifolium C.Agardh 1820 | 0.65 | <0.01 | 1.82 | 1.18 | 0.05 |
| Halodule uninervis (Forssk.) Boiss. | 5.84 | 0.01 | 1.82 | 10.64 | 0.42 |
| Insects | 0.65 | <0.01 | 1.69 | 1.19 | 0.01 |
| Fam. Blattidae | 0.65 | <0.01 | 1.82 | 1.19 | 0.05 |
| Parasites | 23.38 | 0.03 | 10.17 | 255.35 | 2.10 |
| Phylum Nematoda | 20.78 | 0.02 | 7.27 | 151.27 | 5.98 |
| Class Cestoda | 2.60 | 0.01 | 3.64 | 9.48 | 0.37 |
| Abiotic substances | 0.65 | 0.14 | 1.69 | 1.44 | 0.01 |
| Hook and line | 0.65 | 0.14 | 1.82 | 1.44 | 0.06 |
| Male %IRI (n = 27) | Female %IRI (n = 32) | Sex-Combined %IRI (n = 59) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content Items | 0+ yr | 1+ yr | 2+ yr | All | 0+ yr | 1+ yr | 2+ yr | All | 0+ yr | 1+ yr | 2+ yr | All |
| Cephalopods | 0.18 | 0.42 | 6.83 | 0.58 | 0 | 0 | 0 | 0 | 0.04 | 0.07 | 3.88 | 0.18 |
| Octopus cyaneus | 0.59 | 0 | 0 | 0.26 | 0 | 0 | 0 | 0 | 0.16 | 0 | 0 | 0.06 |
| Fam. Sepiidae | 0 | 0 | 14.83 | 0.73 | 0 | 0 | 0 | 0 | 0 | 0 | 9.97 | 0.18 |
| Cephalopod remains | 0 | 1.25 | 0 | 0.20 | 0 | 0 | 0 | 0 | 0 | 0.29 | 0 | 0.05 |
| Elasmobranchs | 0 | 6.04 | 0 | 0.56 | 0 | 14.70 | 85.71 | 5.65 | 0 | 9.40 | 22.17 | 2.84 |
| Maculabatis randalli | 0 | 0 | 0 | 0 | 0 | 0 | 57.98 | 2.99 | 0 | 0 | 24.51 | 0.84 |
| Fam. Dasyatidae | 0 | 7.67 | 0 | 1.47 | 0 | 0 | 0 | 0 | 0 | 2.82 | 0 | 0.39 |
| Fam. Myliobatidae | 0 | 0 | 0 | 0 | 0 | 33.5 | 17.02 | 9.07 | 0 | 12.56 | 4.0 | 2.64 |
| Ord. Myliobatiformes | 0 | 1.42 | 0 | 0.24 | 0 | 0 | 0 | 0 | 0 | 0.35 | 0 | 0.06 |
| Teleosts | 98.93 | 87.33 | 88.61 | 96.61 | 93.17 | 76.77 | 14.29 | 90.42 | 97.02 | 83.79 | 70.97 | 93.81 |
| Saurida tumbil | 0 | 0 | 0 | 0 | 0 | 6.05 | 25.0 | 3.57 | 0 | 2.14 | 8.0 | 1.02 |
| Saurida spp. | 1.76 | 0 | 0 | 0.62 | 0 | 6.03 | 0 | 1.12 | 0.15 | 2.24 | 0 | 0.97 |
| Grammoplites suppositus | 0 | 0 | 0 | 0 | 8.46 | 0 | 0 | 3.27 | 2.61 | 0 | 0 | 0.95 |
| Platycephalus indicus | 0 | 45.50 | 0 | 8.60 | 17.0 | 0 | 0 | 6.58 | 5.24 | 16.07 | 0 | 8.34 |
| Epinephelus coioides | 0 | 10.91 | 0 | 2.12 | 0 | 0 | 0 | 0 | 0 | 4.09 | 0 | 0.57 |
| Epinephelus spp. | 0 | 0 | 46.8 | 2.84 | 0 | 0 | 0 | 0 | 0 | 0 | 24.97 | 0.76 |
| Sillago sihama | 0 | 0 | 0 | 0 | 0 | 1.42 | 0 | 0.36 | 0 | 0.69 | 0 | 0.11 |
| Megalaspis cordyla | 1.68 | 0 | 0 | 0.60 | 0 | 0 | 0 | 0 | 0.43 | 0 | 0 | 0.15 |
| Lutjanus ehrenbergii | 0 | 4.53 | 0 | 0.85 | 0 | 0 | 0 | 0 | 0 | 1.58 | 0 | 0.22 |
| Gerres spp. | 0 | 1.55 | 0 | 0.26 | 0 | 0 | 0 | 0 | 0 | 0.40 | 0 | 0.06 |
| Acanthopagrus bifasciatus | 0 | 0 | 6.19 | 0.28 | 0 | 1.96 | 0 | 0.38 | 0 | 0.77 | 4.41 | 0.36 |
| Lethrinus nebulosus | 0 | 0 | 0 | 0 | 0 | 1.17 | 0 | 0.26 | 0 | 0.50 | 0 | 0.08 |
| Nemipterus japonicus | 1.44 | 0 | 0 | 0.52 | 0 | 0 | 0 | 0 | 0.37 | 0 | 0 | 0.13 |
| Otolithes ruber | 0 | 0 | 0 | 0 | 1.04 | 0 | 0 | 0.39 | 0.30 | 0 | 0 | 0.12 |
| Siganus canaliculatus | 4.40 | 0 | 0 | 1.44 | 0 | 0 | 0 | 0 | 1.10 | 0 | 0 | 0.39 |
| Pseudorhombus spp. | 1.35 | 0 | 0 | 0.57 | 0 | 0 | 0 | 0 | 0.36 | 0 | 0 | 0.14 |
| Fam. Platycephalidae | 2.54 | 2.24 | 0 | 2.84 | 9.13 | 0 | 0 | 3.51 | 6.34 | 0.68 | 0 | 3.46 |
| Fam. Serranidae | 0 | 0 | 0 | 0 | 0 | 3.60 | 0 | 0.66 | 0 | 1.32 | 0 | 0.19 |
| Fam. Scombridae | 0.88 | 0 | 0 | 0.35 | 0 | 0 | 0 | 0 | 0.23 | 0 | 0 | 0.09 |
| Fish remains | 82.56 | 6.24 | 22.28 | 66.09 | 57.07 | 26.80 | 0 | 59.48 | 78.56 | 27.15 | 16.48 | 69.06 |
| Crustaceans | 0.27 | 0.47 | 0 | 0.19 | 6.45 | 0 | 0 | 1.78 | 2.47 | 0.09 | 0 | 0.94 |
| Marsupenaeus japonicus | 0 | 0 | 0 | 0 | 1.14 | 0 | 0 | 0.44 | 0.35 | 0 | 0 | 0.13 |
| Penaeus semisulcatus | 0 | 0 | 0 | 0 | 1.16 | 0 | 0 | 0.44 | 0.34 | 0 | 0 | 0.13 |
| Fam. Penaeidae | 0.85 | 1.42 | 0 | 1.15 | 3.32 | 0 | 0 | 1.25 | 2.13 | 0.35 | 0 | 1.33 |
| Crustacean remains | 0 | 0 | 0 | 0 | 0.40 | 0 | 0 | 0.15 | 0.11 | 0 | 0 | 0.05 |
| Bivalves | 0 | 0 | 0 | 0 | 0 | 0.22 | 0 | 0.03 | 0 | 0.07 | 0 | 0.01 |
| Bivalve remains | 0 | 0 | 0 | 0 | 0 | 0.51 | 0 | 0.15 | 0 | 0.28 | 0 | 0.05 |
| Plants | 0 | 0 | 0 | 0 | 0 | 2.25 | 0 | 0.63 | 0 | 0.71 | 0 | 0.10 |
| Sargassum angustifolium | 0 | 0 | 0 | 0 | 0 | 0.51 | 0 | 0.15 | 0 | 0.28 | 0 | 0.05 |
| Halodule uninervis | 0 | 0 | 0 | 0 | 0 | 4.61 | 0 | 1.33 | 0 | 2.52 | 0 | 0.42 |
| Insects | 0 | 0 | 0 | 0 | 0 | 0.23 | 0 | 0.03 | 0 | 0.07 | 0 | 0.01 |
| Fam. Blattidae | 0 | 0 | 0 | 0 | 0 | 0.52 | 0 | 0.15 | 0.28 | 0.05 | ||
| Parasites | 0.62 | 5.47 | 4.56 | 2.06 | 0.24 | 5.84 | 0 | 1.42 | 0.43 | 5.79 | 2.98 | 2.10 |
| Phylum Nematoda | 1.98 | 17.26 | 0 | 7.56 | 0 | 13.32 | 0 | 3.83 | 0.56 | 22.63 | 0 | 5.98 |
| Class Cestoda | 0 | 0 | 9.90 | 0.40 | 0.79 | 0 | 0 | 0.30 | 0.22 | 0 | 7.66 | 0.37 |
| Abiotic substances | 0 | 0 | 0 | 0 | 0.14 | 0 | 0 | 0.04 | 0.04 | 0 | 0 | 0.01 |
| Hook and line | 0 | 0 | 0 | 0 | 0.49 | 0 | 0 | 0.18 | 0.14 | 0 | 0 | 0.06 |
| Sex and Age Class | Male | Female | All Male | All Female | Sex Combined | TrL | BA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0+ | 1+ | 2+ | 0+ | 1+ | 2+ | 0+ | 1+ | 2+ | Lower Taxa (Order to species) | Group Class | ||||
| M 0+, n = 16 | - | - | - | - | - | - | - | - | - | - | - | 4.76 | 0.05 | 0.03 |
| M 1+, n = 8 | 0.007 | - | - | - | - | - | - | - | - | - | - | 4.71 | 0.21 | 0.16 |
| M 2+, n = 3 | 0 | 0 | - | - | - | - | - | - | - | - | - | 4.40 | 0.08 | 0.16 |
| F 0+, n = 20 | 0.04 | 0.53 | 0 | - | - | - | - | - | - | - | - | 4.56 | 0.13 | 0.10 |
| F 1+ yr, n = 9 | 0.12 | 0 | 0.003 | 0 | - | - | - | - | - | - | - | 4.61 | 0.10 | 0.23 |
| F, 2+ yr, n = 3 | 0 | 0 | 0 | 0 | 0.08 | - | - | - | - | - | - | 5.01 | 0.06 | 0.13 |
| All male, n = 27 | - | - | - | - | - | - | - | - | - | - | - | 4.70 | 0.12 | 0.07 |
| All female, n = 32 | - | - | - | - | - | - | 0.23 | - | - | - | - | 4.62 | 0.16 | 0.20 |
| All 0+, n = 36 | - | - | - | - | - | - | - | - | - | - | - | 4.65 | 0.10 | 0.07 |
| All 1+, n = 17 | - | - | - | - | - | - | - | - | 0.34 | - | - | 4.65 | 0.16 | 0.22 |
| All 2+, n = 6 | - | - | - | - | - | - | - | - | 0 | 0.04 | - | 4.70 | 0.15 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.H.; Nazeer, Z.; Panickan, P.; Lin, Y.-J.; Qasem, A.; Rabaoui, L.J.; Qurban, M.A. Stomach Content Analysis for Juvenile Great Hammerhead Sharks Sphyrna mokarran (Rüppell, 1837) from the Arabian Gulf. Fishes 2022, 7, 359. https://doi.org/10.3390/fishes7060359
Hsu HH, Nazeer Z, Panickan P, Lin Y-J, Qasem A, Rabaoui LJ, Qurban MA. Stomach Content Analysis for Juvenile Great Hammerhead Sharks Sphyrna mokarran (Rüppell, 1837) from the Arabian Gulf. Fishes. 2022; 7(6):359. https://doi.org/10.3390/fishes7060359
Chicago/Turabian StyleHsu, Hua Hsun, Zahid Nazeer, Premlal Panickan, Yu-Jia Lin, Ali Qasem, Lotfi Jilani Rabaoui, and Mohammad Ali Qurban. 2022. "Stomach Content Analysis for Juvenile Great Hammerhead Sharks Sphyrna mokarran (Rüppell, 1837) from the Arabian Gulf" Fishes 7, no. 6: 359. https://doi.org/10.3390/fishes7060359
APA StyleHsu, H. H., Nazeer, Z., Panickan, P., Lin, Y. -J., Qasem, A., Rabaoui, L. J., & Qurban, M. A. (2022). Stomach Content Analysis for Juvenile Great Hammerhead Sharks Sphyrna mokarran (Rüppell, 1837) from the Arabian Gulf. Fishes, 7(6), 359. https://doi.org/10.3390/fishes7060359