Winter Behavior of Juvenile Brown Trout in a Changing Climate: How Do Light and Ice Cover Affect Encounters with Instream Predators?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Fish
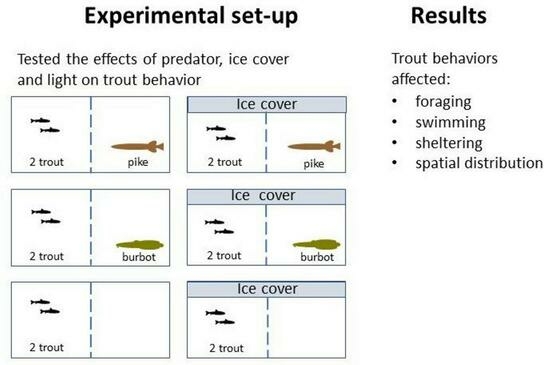
2.2. Experimental Design
2.3. Data Collection and Statistical Analyses
3. Results
3.1. Foraging Behavior
3.2. Aggression
3.3. Activity and Shelter Use
3.4. Position of Trout in the Flumes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Source of Variation | F | df | p |
|---|---|---|---|---|
| Foraging (Y/N) | Predator | 4.35 | 2, 127 | 0.02 |
| binomial distribution | Ice cover | 0.01 | 1, 127 | 0.91 |
| Light | 14.14 | 1, 127 | <0.001 | |
| Predator x Ice cover | 0.44 | 2, 127 | 0.65 | |
| Predator x Light | 0.61 | 2, 127 | 0.55 | |
| Ice cover x Light | 0.51 | 1, 127 | 0.48 | |
| Predator x Ice cover x Light | 0.34 | 2, 127 | 0.72 | |
| Mass | 0.79 | 1, 127 | 0.38 | |
| Foraging rate (continuous) | Predator | 1.96 | 2, 127 | 0.15 |
| linear distribution | Ice cover | 3.12 | 1, 127 | 0.08 |
| Light | 53.55 | 1, 127 | <0.001 | |
| Predator x Ice cover | 0.07 | 2, 127 | 0.94 | |
| Predator x Light | 1.94 | 2, 127 | 0.15 | |
| Ice cover x Light | 6.25 | 1, 127 | 0.01 | |
| Predator x Ice cover x Light | 0.43 | 2, 127 | 0.65 | |
| Mass | 4.49 | 1, 127 | 0.04 | |
| Aggression (Y/N) | Predator | 1.00 | 2, 131 | 0.37 |
| binomial distribution | Ice cover | 0.34 | 1, 131 | 0.56 |
| Feeding | 6.53 | 1, 131 | 0.01 | |
| Predator x Ice cover | 0.79 | 2, 131 | 0.46 | |
| Predator x Feeding | 0.26 | 2, 131 | 0.77 | |
| Ice cover x Feeding | 0.01 | 1, 131 | 0.93 | |
| Predator x Ice cover x Feeding | 0.16 | 2, 131 | 0.85 | |
| Mass | 3.62 | 1, 131 | 0.06 | |
| Swimming activity (proportion) | Predator | 7.29 | 2, 271 | <0.001 |
| linear distribution | Ice cover | 0.01 | 1, 271 | 0.91 |
| Light | 25.81 | 1, 271 | <0.001 | |
| Feeding | 3.02 | 1, 271 | 0.08 | |
| Predator x Ice cover | 0.31 | 2, 271 | 0.73 | |
| Predator x Light | 6.52 | 2, 271 | 0.002 | |
| Predator x Feeding | 0.01 | 2, 271 | 0.96 | |
| Ice cover x Light | 0.86 | 1, 271 | 0.36 | |
| Ice cover x Feeding | 0.33 | 1, 271 | 0.57 | |
| Light x Feeding | 0.02 | 1, 271 | 0.88 | |
| Predator x Ice cover x Light | 0.20 | 2, 271 | 0.99 | |
| Predator x Light x Feeding | 0.13 | 2, 271 | 0.88 | |
| Ice cover x Light x Feeding | 0.18 | 1, 271 | 0.67 | |
| Predator x Ice cover x Feeding | 0.78 | 2, 271 | 0.46 | |
| Predator x Ice cover x Light x Feeding | 0.08 | 2, 271 | 0.92 | |
| Mass | 8.72 | 1, 271 | 0.003 | |
| Shelter use (proportion) | Predator | 3.70 | 2, 271 | 0.03 |
| linear distribution | Ice cover | 2.52 | 1, 271 | 0.11 |
| Light | 11.35 | 1, 271 | <0.001 | |
| Feeding | 0.72 | 1, 271 | 0.40 | |
| Predator x Ice cover | 1.88 | 2, 271 | 0.16 | |
| Predator x Light | 1.05 | 2, 271 | 0.35 | |
| Predator x Feeding | 0.13 | 2, 271 | 0.88 | |
| Ice cover x Light | 0.77 | 1, 271 | 0.38 | |
| Ice cover x Feeding | 0.23 | 1, 271 | 0.63 | |
| Light x Feeding | 0.33 | 1, 271 | 0.57 | |
| Predator x Ice cover x Light | 1.10 | 2, 271 | 0.34 | |
| Predator x Light x Feeding | 0.12 | 2, 271 | 0.88 | |
| Ice cover x Light x Feeding | 0.00 | 1, 271 | 0.97 | |
| Predator x Ice cover x Feeding | 0.27 | 2, 271 | 0.76 | |
| Predator x Ice cover x Light x Feeding | 0.03 | 2, 271 | 0.98 | |
| Mass | 8.94 | 1, 271 | 0.003 | |
| Distance from predator arena (continuous) | Predator | 1.54 | 2, 267 | 0.22 |
| linear distribution | Ice cover | 13.48 | 1, 267 | <0.001 |
| Light | 8.16 | 1, 267 | 0.01 | |
| Feeding | 0.59 | 1, 267 | 0.44 | |
| Predator x Ice cover | 1.25 | 2, 267 | 0.29 | |
| Predator x Light | 2.55 | 2, 267 | 0.08 | |
| Predator x Feeding | 0.15 | 2, 267 | 0.86 | |
| Ice cover x Light | 0.01 | 1, 267 | 0.82 | |
| Ice cover x Feeding | 0.00 | 1, 267 | 0.98 | |
| Light x Feeding | 0.06 | 1, 267 | 0.81 | |
| Predator x Ice cover x Light | 2.84 | 2, 267 | 0.06 | |
| Predator x Light x Feeding | 0.09 | 2, 267 | 0.92 | |
| Ice cover x Light x Feeding | 0.36 | 1, 267 | 0.55 | |
| Predator x Ice cover x Feeding | 0.35 | 2, 267 | 0.70 | |
| Predator x Ice cover x Light x Feeding | 0.64 | 2, 267 | 0.53 | |
| Mass | 13.34 | 1, 267 | <0.001 | |
| Distance between trout (continuous) | Predator | 0.18 | 2, 265 | 0.83 |
| linear distribution | Ice cover | 2.46 | 1, 265 | 0.12 |
| Light | 2.76 | 1, 265 | 0.10 | |
| Feeding | 0.18 | 1, 265 | 0.67 | |
| Predator x Ice cover | 6.60 | 2, 265 | <0.001 | |
| Predator x Light | 0.58 | 2, 265 | 0.57 | |
| Predator x Feeding | 0.29 | 2, 265 | 0.75 | |
| Ice cover x Light | 4.72 | 1, 265 | 0.03 | |
| Ice cover x Feeding | 0.16 | 1, 265 | 0.69 | |
| Light x Feeding | 0.01 | 1, 265 | 0.92 | |
| Predator x Ice cover x Light | 2.04 | 2, 265 | 0.13 | |
| Predator x Light x Feeding | 0.21 | 2, 265 | 0.81 | |
| Ice cover x Light x Feeding | 0.00 | 1, 265 | 0.98 | |
| Predator x Ice cover x Feeding | 1.78 | 2, 265 | 0.17 | |
| Predator x Ice cover x Light x Feeding | 0.75 | 2, 265 | 0.47 | |
| Mass | 1.21 | 1, 265 | 0.27 |
References
- McKenzie, D.J.; Geffroy, B.; Farrell, A.P. Effects of global warming on fishes and fisheries. J. Fish Biol. 2021, 98, 1489–1492. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Heino, J.; Erkinaro, J.; Huusko, A.; Luoto, M. Climate change effects on freshwater fishes, conservation and management. In Conservation of Freshwater Fishes; Closs, G.P., Krkosek, M., Olden, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 76–106. [Google Scholar] [CrossRef]
- Falvo, C.A.; Koons, D.N.; Aubry, L.M. Seasonal climate effects on the survival of a hibernating mammal. Ecol. Evol. 2019, 9, 3756–3769. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Kerry, J.T.; Fraser, C.I. Rapid winter warming could disrupt coastal marine fish community structure. Nat. Clim. Change 2020, 10, 862–867. [Google Scholar] [CrossRef]
- Brown, R.D. Northern hemisphere snow cover variability and change, 1915–97. J. Clim. 2000, 13, 2339–2355. [Google Scholar] [CrossRef]
- Magnuson, J.J.; Robertson, D.M.; Benson, B.J.; Wynne, R.H.; Livingstone, D.M.; Arai, T.; Assel, R.A.; Barry, R.G.; Card, V.V.; Kuusisto, E.; et al. Historical trends in lake and river ice cover in the northern hemisphere. Science 2000, 289, 1743–1746. [Google Scholar] [CrossRef]
- Post, E.; Forchhammer, M.C.; Bret-Harte, M.S.; Callaghan, T.V.; Christensen, T.R.; Elberling, B.; Fox, A.D.; Gilg, O.; Hik, D.S.; Høye, T.T.; et al. Ecological dynamics across the Arctic associated with recent climate change. Science 2009, 325, 1355–1358. [Google Scholar] [CrossRef]
- Choi, G.; Robinson, D.A.; Kang, S. Changing northern hemisphere snow seasons. J. Clim. 2010, 23, 5305–5310. [Google Scholar] [CrossRef]
- Callaghan, T.V.; Johansson, M.; Brown, R.D.; Groisman, P.; Labba, N.; Radionov, V.; Barry, R.; Bulygina, O.; Essery, R.; Frolov, D.; et al. The changing face of Arctic snow cover: A synthesis of observed and projected changes. Ambio 2011, 40, 17–31. [Google Scholar] [CrossRef]
- Hurst, T.P. Causes and consequences of winter mortality in fishes. J. Fish. Biol. 2007, 71, 315–345. [Google Scholar] [CrossRef]
- Huusko, A.; Greenberg, L.; Stickler, M.; Linnansaari, T.; Nykänen, M.; Vehanen, T.; Koljonen, S.; Louhi, P.; Alfredsen, K. Life in the ice lane: The winter ecology of stream salmonids. River Res. Appl. 2007, 23, 469–491. [Google Scholar] [CrossRef]
- Harvey, B.C.; Nakamoto, R.J. Seasonal and among-stream variation in predator encounter rates for fish prey. Trans. Am. Fish. Soc. 2013, 142, 621–627. [Google Scholar] [CrossRef]
- Webb, P.W. Temperature effects on acceleration of rainbow trout, Salmo gairdneri. J. Fish. Res. Board Canada 1978, 35, 1417–1422. [Google Scholar] [CrossRef]
- Bennett, A.F. Thermal dependence of locomotor capacity. Am. J Physiol. Regul. Integr. Comp. Physiol. 1990, 259, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Guderley, H. Metabolic responses to low temperature in fish muscle. Biol. Rev. Camb. Philos. Soc. 2004, 79, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A. Environment and plasticity of myogenesis in teleost fish. J. Exp. Biol. 2006, 209, 2249–2264. [Google Scholar] [CrossRef]
- Valdimarsson, S.K.; Metcalfe, N.B. Shelter selection in juvenile Atlantic salmon, or why do salmon seek shelter in winter? J. Fish Biol. 1998, 52, 42–49. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Ecology of Atlantic Salmon and Brown Trout: Habitat as a Template for Life Histories; Springer: Dordrecht, The Netherlands, 2011; ISBN 978-94-007-1188-4. [Google Scholar]
- Enefalk, Å.; Watz, J.; Greenberg, L.; Bergman, E. Winter sheltering by juvenile brown trout (Salmo trutta)—Effects of stream wood and an instream ectothermic predator. Freshw. Biol. 2017, 62, 111–118. [Google Scholar] [CrossRef]
- Filipsson, K.; Bergman, E.; Österling, M.; Erlandsson, A.; Greenberg, L.; Watz, J. Effects of temperature and a piscivorous fish on diel winter behaviour of juvenile brown trout (Salmo trutta). Freshw. Biol. 2019, 64, 1797–1805. [Google Scholar] [CrossRef]
- Orpwood, J.E.; Griffiths, S.W.; Armstrong, J.D. Effects of food availability on temporal activity patterns and growth of Atlantic salmon. J. Anim. Ecol. 2006, 75, 677–685. [Google Scholar] [CrossRef]
- Heggenes, J.; Krog, O.M.W.; Lindås, O.R.; Dokk, J.G. Homeostatic behavioural responses in a changing environment: Brown trout (Salmo trutta) become nocturnal during winter. J. Anim. Ecol. 1993, 62, 295–308. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Fraser, N.H.C.; Burns, M.D. Food availability and the nocturnal vs. diurnal foraging trade-off in juvenile salmon. J. Anim. Ecol. 1999, 68, 371–381. [Google Scholar] [CrossRef]
- Nykänen, M.; Huusko, A.; Lahti, M. Changes in movement, range and habitat preferences of adult grayling from late summer to early winter. J. Fish Biol. 2004, 64, 1386–1398. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Steele, G.I. Changing nutritional status causes a shift in the balance of nocturnal to diurnal activity in European minnows. Funct. Ecol. 2001, 15, 304–309. [Google Scholar] [CrossRef]
- David, B.O.; Closs, G.P. Seasonal variation in diel activity and microhabitat use of an endemic New Zealand stream-dwelling galaxiid fish. Freshw. Biol. 2003, 48, 1765–1781. [Google Scholar] [CrossRef]
- Heggenes, J.; Borgstrøm, R. Effect of mink, Mustela vison Schreber, predation on cohorts of juvenile Atlantic salmon, Salmo salar L., and brown trout, S. trutta L., in three small streams. J. Fish Biol. 1988, 33, 885–894. [Google Scholar] [CrossRef]
- Prowse, T.D. River-ice ecology. II: Biological aspects. J. Cold Reg. Eng. 2001, 15, 17–33. [Google Scholar] [CrossRef]
- Finstad, A.G.; Forseth, T.; Næsje, T.F.; Ugedal, O. The importance of ice cover for energy turnover in juvenile Atlantic salmon. J. Anim. Ecol. 2004, 73, 959–966. [Google Scholar] [CrossRef]
- Linnansaari, T.; Alfredsen, K.; Stickler, M.; Arnekleiv, J.O.; Harby, A.; Cunjak, R.A. Does ice matter? Site fidelity and movements by Atlantic salmon (Salmo salar L.) parr during winter in a substrate enhanced river reach. River Res. Appl. 2008, 25, 773–787. [Google Scholar] [CrossRef]
- Brown, R.S.; Hubert, W.A.; Daly, S.F. A primer on winter, ice, and fish: What fisheries biologists should know about winter ice processes and stream-dwelling fish. Fisheries 2011, 36, 8–26. [Google Scholar] [CrossRef]
- Hedger, R.D.; Næsje, T.F.; Fiske, P.; Ugedal, O.; Finstad, A.G.; Thorstad, E.B. Ice-dependent winter survival of juvenile Atlantic salmon. Ecol. Evol. 2013, 3, 523–535. [Google Scholar] [CrossRef]
- Watz, J.; Bergman, E.; Piccolo, J.J.; Greenberg, L. Ice cover affects the growth of a stream-dwelling fish. Oecologia 2016, 181, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Härkönen, L.; Louhi, P.; Huusko, R.; Huusko, A. Wintertime growth in Atlantic salmon under changing climate: The importance of ice cover for individual growth dynamics. Can. J. Fish. Aquat. Sci. 2021, 78, 1479–1485. [Google Scholar] [CrossRef]
- Watz, J.; Bergman, E.; Calles, O.; Enefalk, Å.; Gustafsson, S.; Hagelin, A.; Nilsson, A.; Norrgård, J.; Nyqvist, D.; Österling, E.M.; et al. Ice cover alters the behavior and stress level of brown trout Salmo trutta. Behav. Ecol. 2015, 26, 820–827. [Google Scholar] [CrossRef]
- Watz, J.; Bergman, E.; Piccolo, J.J.; Greenberg, L. 2013. Effects of ice cover on the diel behaviour and ventilation rate of juvenile brown trout. Freshw. Biol. 2013, 58, 2325–2332. [Google Scholar] [CrossRef]
- Jakober, M.J.; McMahon, T.E.; Thurow, R.F.; Clancy, C.G. Role of stream ice on fall and winter movements and habitat use by bull trout and cutthroat trout in Montana headwater streams. Trans. Am. Fish. Soc. 1998, 127, 223–235. [Google Scholar] [CrossRef]
- Helland, I.P.; Finstad, A.G.; Forseth, T.; Hesthagen, T.; Ugedal, O. 2011. Ice-cover effects on competitive interactions between two fish species. J. Anim. Ecol. 2011, 80, 539–547. [Google Scholar] [CrossRef]
- Watz, J. Stress responses of juvenile brown trout under winter conditions in a laboratory stream. Hydrobiologia 2017, 802, 131–140. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Preisser, E.L.; Bolnick, D.I.; Benard, M.F. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 2005, 86, 501–509. [Google Scholar] [CrossRef]
- Tolonen, A.; Kjellman, J.; Lappalainen, J. Diet overlap between burbot (Lota lota (L.)) and whitefish (Coregonus lavaretus (L.)) in a subarctic lake. Ann. Zool. Fenn. 1999, 36, 205–214. [Google Scholar]
- Kahilainen, K.; Lehtonen, H. Piscivory and prey selection of four predator species in a whitefish dominated subarctic lake. J. Fish. Biol. 2003, 63, 659–672. [Google Scholar] [CrossRef]
- Hyvärinen, P.; Vehanen, T. Effect of brown trout body size on post-stocking survival and pike predation. Ecol. Freshw. Fish. 2004, 13, 77–84. [Google Scholar] [CrossRef]
- Vehanen, T.; Hamari, S. Predation threat affects behaviour and habitat use by hatchery brown trout (Salmo trutta L.) juveniles. Hydrobiologia 2004, 525, 229–237. [Google Scholar] [CrossRef]
- Hawkins, L.A.; Armstrong, J.D.; Magurran, A.E. Predator-induced hyperventilation in wild and hatchery Atlantic salmon fry. J. Fish. Biol. 2004, 65, 88–100. [Google Scholar] [CrossRef]
- Hackney, P.A. Ecology of the Burbot (Lota lota) with Special Reference to Its Role in the Lake Opeongo Fish Community. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 1973. [Google Scholar]
- Öhlund, G.; Hedström, P.; Norman, S.; Hein, C.L.; Englund, G. Temperature dependence of predation depends on the relative performance of predators and prey. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142254. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Hall: Upper Sadle River, NJ, USA, 2010. [Google Scholar]
- Littell, R.C.; Pendergast, J.; Natarajan, R. Modelling covariance structure in the analysis of repeated measures data. Stat. Med. 2000, 19, 1793–1819. [Google Scholar] [CrossRef]
- Filipsson, K.; Bergman, E.; Greenberg, L.; Österling, M.; Watz, J.; Erlandsson, A. Temperature and predator-mediated regulation of plasma cortisol and brain gene expression in juvenile brown trout (Salmo trutta). Front. Zool. 2020, 17, 25. [Google Scholar] [CrossRef]
- Pulliainen, E.; Korhonen, K. Seasonal changes in condition indices in adult mature and non-maturing burbot, Lota lota (L.), in the north-eastern Bothnian bay, northern Finland. J. Fish Biol. 1990, 36, 251–259. [Google Scholar] [CrossRef]
- Edsall, T.A.; Kennedy, G.W.; Horns, W.H. Distribution, abundance, and resting microhabitat of burbot on Julian’s Reef, southwestern Lake Michigan. Trans. Am. Fish. Soc. 1993, 122, 560–574. [Google Scholar] [CrossRef]
- Carl, L.M. Sonic tracking of burbot in Lake Opeongo, Ontario. Trans. Am. Fish. Soc. 1995, 124, 77–83. [Google Scholar] [CrossRef]
- Binner, M.; Kloas, W.; Hardewig, I. Energy allocation in juvenile roach and burbot under different temperature and feeding regimes. Fish Physiol. Biochem. 2008, 34, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Watz, J.; Piccolo, J.J.; Bergman, E.; Greenberg, L. Day and night drift-feeding by juvenile salmonids at low water temperatures. Environ. Biol. Fishes 2014, 97, 505–513. [Google Scholar] [CrossRef]
- Vilhunen, S.; Hirvonen, H. Innate antipredator responses of Arctic charr (Salvelinus alpinus) depend on predator species and their diet. Behav. Ecol. Sociobiol. 2003, 55, 1–10. [Google Scholar] [CrossRef]
- Rosell, F.; Holtan, L.B.; Thorsen, J.G.; Heggenes, J. Predator-naïve brown trout (Salmo trutta) show antipredator behaviours to scent from an introduced piscivorous mammalian predator fed conspecifics. Ethology 2013, 119, 303–308. [Google Scholar] [CrossRef]
- Mikheev, V.N.; Wanzenböck, J.; Pasternak, A.F. Effects of predator-induced visual and olfactory cues on 0+ perch (Perca fluviatilis L.) foraging behaviour. Ecol. Freshw. Fish 2006, 15, 111–117. [Google Scholar] [CrossRef]
- Prowse, T.D. River-ice ecology. I: Hydrologic, geomorphic, and water-quality aspects. J. Cold Reg. Eng. 2001, 127, 201–208. [Google Scholar] [CrossRef]
- Smol, J.P.; Wolfe, A.P.; Birks, H.J.B.; Douglas, M.S.V.; Jones, V.J.; Korhola, A.; Pienitz, R.; Ruhland, K.; Sorvari, S.; Antoniades, D.; et al. Climate-driven regime shifts in the biological communities of Arctic lakes. Proc. Natl. Acad. Sci. USA 2005, 102, 4397–4402. [Google Scholar] [CrossRef] [PubMed]
- Kausrud, K.L.; Mysterud, A.; Steen, H.; Vik, J.O.; Østbye, E.; Cazelles, B.; Framstad, E.; Eikeset, A.M.; Mysterud, I.; Solhøy, T.; et al. Linking climate change to lemming cycles. Nature 2008, 456, 93–97. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipsson, K.; Åsman, V.; Greenberg, L.; Österling, M.; Watz, J.; Bergman, E. Winter Behavior of Juvenile Brown Trout in a Changing Climate: How Do Light and Ice Cover Affect Encounters with Instream Predators? Fishes 2023, 8, 521. https://doi.org/10.3390/fishes8100521
Filipsson K, Åsman V, Greenberg L, Österling M, Watz J, Bergman E. Winter Behavior of Juvenile Brown Trout in a Changing Climate: How Do Light and Ice Cover Affect Encounters with Instream Predators? Fishes. 2023; 8(10):521. https://doi.org/10.3390/fishes8100521
Chicago/Turabian StyleFilipsson, Karl, Veronika Åsman, Larry Greenberg, Martin Österling, Johan Watz, and Eva Bergman. 2023. "Winter Behavior of Juvenile Brown Trout in a Changing Climate: How Do Light and Ice Cover Affect Encounters with Instream Predators?" Fishes 8, no. 10: 521. https://doi.org/10.3390/fishes8100521
APA StyleFilipsson, K., Åsman, V., Greenberg, L., Österling, M., Watz, J., & Bergman, E. (2023). Winter Behavior of Juvenile Brown Trout in a Changing Climate: How Do Light and Ice Cover Affect Encounters with Instream Predators? Fishes, 8(10), 521. https://doi.org/10.3390/fishes8100521