Effects of Oral Antihistamines on Tear Volume, Tear Stability, and Intraocular Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Treatment Preparation
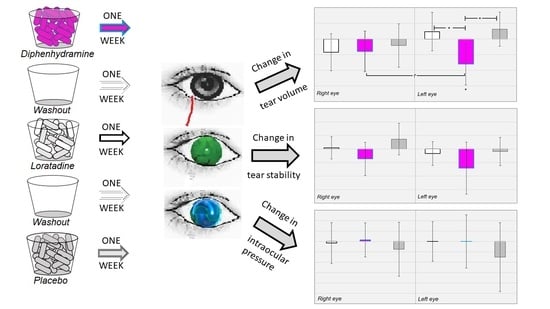
2.3. Experimental Design
| Day 0 | Subjective (eligibility) and objective evaluation. |
| Day 1–7 | Loratadine 10 mg upon waking in AM, placebo at noon, placebo at 6 PM. |
| Day 8 | Objective evaluation. |
| Day 8–14 | Washout period. |
| Day 14 | Objective evaluation. |
| Day 15–21 | Diphenhydramine 25 mg upon waking in AM, at noon, and at 6 PM. |
| Day 22 | Objective evaluation. |
| Day 22–28 | Washout period. |
| Day 28 | Objective evaluation. |
| Day 29–35 | Placebo upon waking in AM, noon, and at 6 PM. |
| Day 36 | Objective evaluation. |
2.4. Objective (Clinical) Measures
2.4.1. Phenol Red Thread Test
2.4.2. Tear Break-Up Time
2.4.3. Intraocular Pressure
2.5. Data Analysis
3. Results
3.1. Descriptive Results
3.2. Changes in Tear Volume
3.3. Changes in Tear Stability
3.4. Changes in Intraocular Pressure
4. Discussion
4.1. Strengths
4.2. Limitations
4.3. Clinical Extensions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nietgen, G.W.; Schmidt, J.; Hese, L.; Honemann, C.W.; Durieeux, M.E. Muscarinic receptor functioning and distribution in the eye: Molecular basis and implications for clinical diagnosis and therapy. Eye 1999, 13, 285–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krinsky, D.L.; Ferreri, S.P.; Hemstreet, B.; Hume, A.L.; Newton, G.D.; Rollins, C.J.; Tietze, K.J. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care, 19th ed.; American Pharmaceutical Association: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Gengo, F.; Gabos, C.; Miller, J.K. The pharmacodynamics of diphenhydramine-induced drowsiness and changes in mental performance. Clin. Pharmacol. Ther. 1989, 13, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Santora, J.; Rozek, S.; Samie, M.R. Diphenhydramine-induced dystonia. Clin. Pharm. 1989, 8, 471. [Google Scholar] [PubMed]
- Roila, F.; Donati, D.; Basurto, C.; Del Favero, A. Diphenhydramine and acute dystonia. Ann. Intern. Med. 1989, 111, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Lavenstein, B.L.; Cantor, F.K. Acute dystonia: An unusual reaction to diphenhydramine. J. Am. Med. Assoc. 1976, 236, 291. [Google Scholar] [CrossRef]
- Brait, K.A.; Zagerman, A.J. Dyskinesias after antihistamine use. N. Engl. J. Med. 1977, 296, 111. [Google Scholar] [CrossRef]
- Englisch, W.; Rehn, D.; Schaffler, K.; Wauschkuhn, C.H. Effects of dimethindene maleate on psychomotor performance in the oculodynamic test compared with placebo and loratadine. Arzneim. Drug Res. 1996, 46, 887–890. [Google Scholar]
- Seedor, J.A.; Lamberts, D.; Bergmann, R.B.; Perry, H.D. Filamentary keratitis associated with diphenhydramine hydrochloride (Benadryl). Am. J. Ophthalmol. 1986, 101, 376–377. [Google Scholar] [CrossRef]
- Terry, M.A. Dry eye in the elderly. Drugs Aging 2001, 18, 101–107. [Google Scholar] [CrossRef]
- Burns, M.; Moskowitz, H. Effects of diphenhydramine and alcohol on skills performance. Eur. J. Clin. Pharmacol. 1980, 17, 259–266. [Google Scholar] [CrossRef]
- Borbely, A.A.; Youmbi-Balderer, G. Effect of diphenhydramine on subjective sleep parameters and on motor activity during bedtime. Int. J. Clin. Pharmacol. Ther. Toxicol. 1988, 26, 392–396. [Google Scholar] [PubMed]
- Carruthers, S.G.; Shoeman, D.W.; Hignite, C.E.; Azarnoff, D.L. Correlation between plasma diphenhydramine level and sedative and antihistamine effects. Clin. Pharmacol. Ther. 1978, 23, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kellerman, D.J. The use of oral antihistamines (OA) in moderate to severe dry eye patients. J. Allergy Clin. Immunol. 2008, 121 (Suppl. 1), S131. [Google Scholar] [CrossRef]
- Ousler, G.W.; Wilcox, K.A.; Gupta, G.; Abelson, M.B. An evaluation of the ocular drying effects of 2 systemic antihistamines: Loratadine and cetirizine hydrochloride. Ann. Allergy Asthma Immunol. 2004, 93, 460–464. [Google Scholar] [CrossRef]
- Welch, D.; Ousler, G.W., III; Nally, L.A.; Abelson, M.B.; Wilcox, K.A. Ocular Drying Associated with Oral Antihistamines (Loratadine) in the Normal Population-an Evaluation of Exaggerated Dose Effect; Sullivan, D.A., Stern, M.E., Tsubota, K., Dartt, D.A., Sullivan, R.M., Bromberg, B.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar] [CrossRef]
- George, W.; Ousler, D.; Workman, A.; Torkildsen, G.L. An open-label, investigator-masked, crossover study of the ocular drying effects of two antihistamines, topical epinastine and systemic loratadine, in adult volunteers with seasonal allergic conjunctivitis. Clin. Ther. 2007, 29, 611–616. [Google Scholar] [CrossRef]
- Razeghinejad, M.R.; Myers, J.S.; Katz, L.J. Iatrogenic glaucoma secondary to medications. Am. J. Med. 2011, 124, 20–25. [Google Scholar] [CrossRef]
- Lachkar, Y.; Bouassida, W. Drug-induced acute angle closure glaucoma. Curr. Opin. Ophthalmol. 2007, 18, 129–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupin, T.; Silverstein, B.; Feitl, M.; Roshe, R.; Becker, B. The Effect of H1-Blocking Antihistamines on Intraocular Pressure in Rabbits. Ophthalmology 1980, 87, 1167–1172. [Google Scholar] [CrossRef]
- Lanzi, C.; Lucarini, L.; Durante, M.; Sgambellone, S.; Pini, A.; Catarinicchia, S.; Łażewska, D.; Kieć-Kononowicz, K.; Stark, H.; Masini, E. Role of Histamine H3 Receptor Antagonists on Intraocular Pressure Reduction in Rabbit Models of Transient Ocular Hypertension and Glaucoma. Int. J. Mol. Sci. 2019, 20, 981. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.M.; Lynch, G.L.; Labelle, P. Effects of oral administration of diphenhydramine on pupil diameter, intraocular pressure, tear production, tear film quality, conjunctival goblet cell density, and corneal sensitivity of clinically normal adult dogs. Am. J. Vet. Res. 2012, 73, 1983–1986. [Google Scholar] [CrossRef] [Green Version]
- Saleh, T.; McDermott, B.; Bates, A.; Ewings, P. Phenol red thread test vs Schirmer’s test: A comparative study. Eye 2006, 20, 913–915. [Google Scholar] [CrossRef]
- Sakamoto, R.; Bennett, E.S.; Henry, V.; Paragina, S.; Narumi, T.; Izumi, Y.; Kamei, Y.; Nagatomi, E.; Miyanaga, Y.; Hamano, H. The phenol red thread tear test: A cross-cultural study. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3510–3514. [Google Scholar] [PubMed]
- McMonnies, C.W. Tear instability importance, mechanisms, validity and reliability of assessment. J. Optom. 2018, 11, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A.; Badouin, C.; Baum, J.; Dogru, M. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Chao, W.; Belmonte, C.; Del Castillo, J.M.B.; Bron, A.J.; Dua, H.S.; Nichols, K.K.; Novack, G.D.; Schrader, S.; Willcox, M.; Wolffsohn, J.S.; et al. Report of the inaugural meeting of the TFOS i2 = initiating innovation series: Targeting the unmet need for dry eye treatment. Ocul Surf. 2016, 14, 264–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downie, L.E.; Keller, P.R.; Vingrys, A.J. An evidence-based analysis of Australian optometrists’ dry eye practices. Optom. Vis. Sci. 2013, 90, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Goldman, H.; Schmidt, T.H. über Applanationstonometrie. Ophthalmologica 1957, 134, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Okafor, K.C.; Brandt, J.D. Measuring intraocular pressure. Curr. Opin. Ophthalmol. 2015, 26, 103–109. [Google Scholar] [CrossRef]
- Moss, S.E.; Klein, R.; Klein, B.E. Prevalence of and risk factors for dry eye syndrome. Arch. Ophthalmol. 2000, 118, 1264–1268. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food & Drug Administration. Drug Approval Package (Claritin), 2004. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/19670s18,20470s16,19658s18,20704s8,20641s9_Claritin.cfm (accessed on 12 May 2020).
- Elflein, J.U.S. Top 10 OTC Allergy Brands by Revenue 2016, 2019. Available online: https://www.statista.com/statistics/296120/top-ten-us-over-the-counter-brands-for-cough-and-cold/ (accessed on 12 May 2020).
- Shahbandeh, M. Leading Allergy Brands in the U.S. 2017, Based on Sales, 2019. Available online: https://www.statista.com/statistics/194773/leading-us-cold-tablet-brands-in-2013-based-on-sales/ (accessed on 12 May 2020).
- Nielsen & Consumer Healthcare Products Association. OTC Research, 2017. CHPA (Consumer Healthcare Products Association). Available online: https://www.chpa.org/OTCResearch.aspx (accessed on 12 May 2020).
- National Center for Biotechnology Information. PubChem Database. Diphenhydramine, CID=3100. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diphenhydramine (accessed on 12 May 2020).
- National Center for Biotechnology Information. PubChem Database. Loratadine, CID=3957. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Loratadine (accessed on 12 May 2020).
- Tomlinson, A.; Blades, K.J.; Pearce, E.I. What does the phenol red thread test actually measure? Optom. Vis. Sci. 2001, 78, 142–146. [Google Scholar] [CrossRef]
- American College of Allergy, Asthma and Immunology. Available online: https://acaai.org/allergies/types/hay-fever-rhinitis (accessed on 8 May 2020).
- Suzuki, S.; Goto, E.; Dogru, M.; Asano-Kato, N.; Matsumoto, Y.; Hara, Y.; Fujishima, H.; Tsubota, K. Tear film layer alterations in allergic conjunctivitis. Cornea 2006, 25, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Benadryl Dosage, 2019. Available online: https://www.benadryl.com/products/benadryl-allergy-ultratab-tablets#ingredients (accessed on 12 May 2020).
- Unisom Useage, 2015. Available online: https://www.unisom.com/our-products/unisom-sleepgels/ (accessed on 12 May 2020).




| PRT Test (mm) | TBUT (s) | IOP (mmHg) | ||||
|---|---|---|---|---|---|---|
| OD | OS | OD | OS | OD | OS | |
| Mean | 21.83 | 21.02 | 8.65 | 8.70 | 15.01 | 14.77 |
| Median | 23.00 | 21.00 | 8.50 | 9.00 | 15.00 | 15.00 |
| St Dev | 5.85 | 5.94 | 2.81 | 2.96 | 3.89 | 3.75 |
| Minimium | 7 | 5 | 3 | 4 | 9 | 9 |
| Maximum | 35 | 37 | 23 | 21 | 21 | 21 |
© 2020 by Brian Foutch, Kyle Sandberg, and Edward Bennett. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foutch, B.K.; Sandberg, K.A.; Bennett, E.S.; Naeger, L.L. Effects of Oral Antihistamines on Tear Volume, Tear Stability, and Intraocular Pressure. Vision 2020, 4, 32. https://doi.org/10.3390/vision4020032
Foutch BK, Sandberg KA, Bennett ES, Naeger LL. Effects of Oral Antihistamines on Tear Volume, Tear Stability, and Intraocular Pressure. Vision. 2020; 4(2):32. https://doi.org/10.3390/vision4020032
Chicago/Turabian StyleFoutch, Brian K., Kyle A. Sandberg, Edward S. Bennett, and Leonard L. Naeger. 2020. "Effects of Oral Antihistamines on Tear Volume, Tear Stability, and Intraocular Pressure" Vision 4, no. 2: 32. https://doi.org/10.3390/vision4020032
APA StyleFoutch, B. K., Sandberg, K. A., Bennett, E. S., & Naeger, L. L. (2020). Effects of Oral Antihistamines on Tear Volume, Tear Stability, and Intraocular Pressure. Vision, 4(2), 32. https://doi.org/10.3390/vision4020032