Clinical and Preclinical Evidence for Adverse Neurodevelopment after Postnatal Zika Virus Infection
Abstract
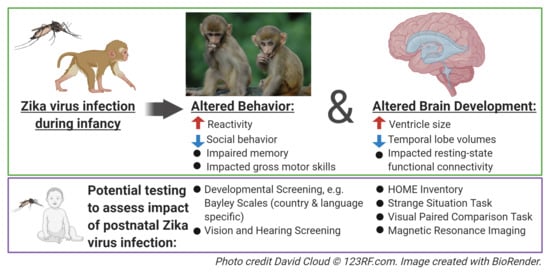
:1. Introduction
2. Clinical Evidence of Postnatal Zika Virus Infection
3. Preclinical Models of Postnatal Zika Virus Infection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias, Í.K.R.; Sobreira, C.L.D.S.; Martins, R.M.G.; Santana, K.F.S.; Lopes, M.D.S.V.; Joventino, E.S.; Viana, M.C.A. Zika virus: A review of the main aspects of this type of arbovirosis. Rev. Soc. Bras. Med. Trop. 2018, 51, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caswell, R.J.; Manavi, K. Emerging sexually transmitted viral infections: 2. Review of Zika virus disease. Int. J. STD AIDS 2018, 29, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Castanha, P.M.S.; Marques, E.T.A. A Glimmer of Hope: Recent Updates and Future Challenges in Zika Vaccine Development. Viruses 2020, 12, 1371. [Google Scholar] [CrossRef]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specficity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Smithburn, K.C. Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. J. Immunol. 1952, 69, 223–234. [Google Scholar] [PubMed]
- Brasil, P.; Pereira, J.P.J.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef]
- Ioos, S.; Mallet, H.P.; Leparc Goffart, I.; Gauthier, V.; Cardoso, T.; Herida, M. Current Zika virus epidemiology and recent epidemics. Med. Mal. Infect. 2014, 44, 302–307. [Google Scholar] [CrossRef]
- Kleber de Oliveira, W.; Cortez-Escalante, J.; De Oliveira, W.T.; do Carmo, G.M.; Henriques, C.M.; Coelho, G.E.; Araújo de França, G.V. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Rice, M.E.; Galang, R.R.; Roth, N.M.; Ellington, S.R.; Moore, C.A.; Valencia-Prado, M.; Ellis, E.M.; Tufa, A.J.; Taulung, L.A.; Alfred, J.M.; et al. Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection—U.S. Territories and Freely Associated States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 858–867. [Google Scholar] [CrossRef] [Green Version]
- Van der Linden, H.; Carvalho, M.D.; van der Linden, V.; Lacerda, K.M.; Pessoa, A.; Carneiro, M.L.; Cordeiro, M.T.; Valente, K.D. Epilepsy Profile in Infants with Congenital Zika Virus Infection. N. Engl. J. Med. 2018, 379, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Van der Linden, V.; Pessoa, A.; Dobyns, W.; Barkovich, A.J.; Junior, H.V.; Filho, E.L.; Ribeiro, E.M.; Leal, M.C.; Coimbra, P.P.; Aragao, M.F.; et al. Description of 13 Infants Born During October 2015–January 2016 with Congenital Zika Virus Infection Without Microcephaly at Birth—Brazil. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- WHO. Zika Virus. Available online: http://www.who.int/news-room/fact-sheets/detail/zika-virus (accessed on 3 October 2020).
- Center for Disease Control Zika Travel Information. Available online: https://wwwnc.cdc.gov/travel/page/zika-information (accessed on 3 October 2020).
- Ruchusatsawat, K.; Wongjaroen, P.; Posanacharoen, A.; Rodriguez-Barraquer, I.; Sangkitporn, S.; Cummings, D.A.T.; Salje, H. Long-term circulation of Zika virus in Thailand: An observational study. Lancet Infect. Dis. 2019, 19, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Sasmono, R.T.; Dhenni, R.; Yohan, B.; Pronyk, P.; Hadinegoro, S.R.; Soepardi, E.J.; Ma’roef, C.N.; Satari, H.I.; Menzies, H.; Hawley, W.A.; et al. Zika Virus Seropositivity in 1–4-Year-Old Children, Indonesia, 2014. Emerg. Infect. Dis. 2018, 24, 1740–1743. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.A. Zika virus. II. Pathogenicity and physical properties. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 521–534. [Google Scholar] [CrossRef]
- Haddow, A.J.; Williams, M.C.; Woodall, J.P.; Simpson, D.I.H.; Goma, L.K.H. Twelve isolations of Zika virus from Aedes (Stegomyia) africanus (Theobald) taken in and above a Uganda forest. Bull. World Health Organ. 1964, 31, 57–69. [Google Scholar]
- Weinbren, M.P.; Williams, M.C. Zika virus: Further isolations in the Zika area, and some studies on the strains isolated. Trans. R. Soc. Trop. Med. Hyg. 1958, 52, 263–268. [Google Scholar] [CrossRef]
- Dang, J.; Tiwari, S.K.; Lichinchi, G.; Qin, Y.; Patil, V.S.; Eroshkin, A.M.; Rana, T.M. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 2016, 19, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Luo, Z.; Zeng, J.; Chen, W.; Foo, S.S.; Lee, S.A.; Ge, J.; Wang, S.; Goldman, S.A.; Zlokovic, B.V.; et al. Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell 2016, 19, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Onorati, M.; Li, Z.; Liu, F.; Sousa, A.M.M.; Nakagawa, N.; Li, M.; Dell’Anno, M.T.; Gulden, F.O.; Pochareddy, S.; Tebbenkamp, A.T.N.; et al. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 2016, 16, 2576–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liu, J.; Zhou, R.; Ding, X.; Zhang, Q.; Zhang, C.; Li, L. Zika virus infected primary microglia impairs NPCs proliferation and differentiation. Biochem. Biophys. Res. Commun. 2018, 497, 619–625. [Google Scholar] [CrossRef]
- Franca, G.V.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Malkki, H. CNS infections: Mouse studies confirm the link between Zika virus infection and microcephaly. Nat. Rev. Neurol. 2016, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Song, H.; Ming, G.L. How does Zika virus cause microcephaly? Genes Dev. 2017, 31, 849–861. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Satterfield-Nash, A.; Kotzky, K.; Allen, J.; Bertolli, J.; Moore, C.A.; Pereira, I.O.; Pessoa, A.; Melo, F.; Santelli, A.C.F.E.S.; Boyle, C.A.; et al. Health and Development at Age 19–24 Months of 19 Children Who Were Born with Microcephaly and Laboratory Evidence of Congenital Zika Virus Infection During the 2015 Zika Virus Outbreak—Brazil, 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1347–1351. [Google Scholar] [CrossRef] [Green Version]
- Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Vasconcelos, Z.; Gabaglia, C.R.; Damasceno, L.; Pone, M.; Abreu de Carvalho, L.M.; Pone, S.M.; Zin, A.A.; et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat. Med. 2019, 25, 1213–1217. [Google Scholar] [CrossRef]
- Einspieler, C.; Utsch, F.; Brasil, P.; Aizawa, C.Y.P.; Peyton, C.; Hasue, R.H.; Genovesi, F.F.; Damasceno, L.; Moreira, M.E.; Adachi, K.; et al. Association of infants exposed to prenatal Zika virus infection with their clinical, neurologic, and developmental status evaluated via the general movement assessment tool. JAMA Netw. Open 2019, 2, 187235. [Google Scholar] [CrossRef] [Green Version]
- Melo, A.; Gama, G.L.; Da Silva Júnior, R.A.; De Assunção, P.L.; Tavares, J.S.; Da Silva, M.B.; Costa, K.N.F.S.; Vânia, M.L.; Evangelista, M.A.; De Amorim, M.M.R. Motor function in children with congenital Zika syndrome. Dev. Med. Child Neurol. 2019, 62, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Frota, L.M.D.C.P.; Sampaio, R.F.; Miranda, J.L.; Brasil, R.M.C.; Gontijo, A.P.B.; Mambrini, J.V.M.; Brandão, M.B.; Mancini, M.C. Children with congenital Zika syndrome: Symptoms, comorbidities and gross motor development at 24 months of age. Heliyon 2020, 6, 04130. [Google Scholar] [CrossRef]
- Deoni, S.C.; O’Muircheartaigh, J.; Elison, J.T.; Walker, L.; Doernberg, E.; Waskiewicz, N.; Dirks, H.; Piryatinsky, I.; Dean, D.C., III; Jumbe, N.L. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct. Funct. 2016, 221, 1189–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girault, J.B.; Cornea, E.; Goldman, B.D.; Knickmeyer, R.C.; Styner, M.; Gilmore, J.H. White matter microstructural development and cognitive ability in the first 2 years of life. Hum. Brain Mapp. 2019, 40, 1195–1210. [Google Scholar] [CrossRef] [Green Version]
- Aragao, M.; Holanda, A.C.; Brainer-Lima, A.M.; Petribu, N.C.L.; Castillo, M.; van der Linden, V.; Serpa, S.C.; Tenorio, A.G.; Travassos, P.T.C.; Cordeiro, M.T.; et al. Nonmicrocephalic Infants with Congenital Zika Syndrome Suspected Only after Neuroimaging Evaluation Compared with Those with Microcephaly at Birth and Postnatally: How Large Is the Zika Virus “Iceberg”? AJNR Am. J. Neuroradiol. 2017, 38, 1427–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulkey, S.B.; Arroyave-Wessel, M.; Peyton, C.; Bulas, D.I.; Fourzali, Y.; Jiang, J.; Russo, S.; McCarter, R.; Msall, M.E.; du Plessis, A.J.; et al. Neurodevelopmental Abnormalities in Children with In Utero Zika Virus Exposure without Congenital Zika Syndrome. JAMA Pediatr. 2020, 174, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.C., 3rd; O’Muircheartaigh, J.; Dirks, H.; Waskiewicz, N.; Lehman, K.; Walker, L.; Han, M.; Deoni, S.C. Modeling healthy male white matter and myelin development: 3 through 60months of age. Neuroimage 2014, 84, 742–752. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Alcauter, S.; Elton, A.; Hernandez-Castillo, C.R.; Smith, J.K.; Ramirez, J.; Lin, W. Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cereb. Cortex 2015, 25, 2919–2928. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Zhu, H.; Giovanello, K.S.; Smith, J.K.; Shen, D.; Gilmore, J.H.; Lin, W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. USA 2009, 106, 6790–6795. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Gouttard, S.; Sharma, A.; Gu, H.; Styner, M.; Lin, W.; Gerig, G.; Gilmore, J.H. Quantitative tract-based white matter development from birth to age 2years. Neuroimage 2012, 61, 542–557. [Google Scholar] [CrossRef] [Green Version]
- Knickmeyer, R.C.; Gouttard, S.; Kang, C.; Evans, D.; Wilber, K.; Smith, J.K.; Hamer, R.M.; Lin, W.; Gerig, G.; Gilmore, J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008, 28, 12176–12182. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhu, Q.; Gao, W.; Chen, Y.; Toh, C.H.; Styner, M.; Gerig, G.; Smith, J.K.; Biswal, B.; Gilmore, J.H. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am. J. Neuroradiol. 2008, 29, 1883–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Muircheartaigh, J.; Dean, D.C., III; Ginestet, C.E.; Walker, L.; Waskiewicz, N.; Lehman, K.; Dirks, H.P., I; Deoni, S.C. White matter development and early cognition in babies and toddlers. Hum. Brain Mapp. 2014, 35, 4475–4487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilmore, J.H.; Shi, F.; Woolson, S.L.; Knickmeyer, R.C.; Short, S.J.; Lin, W.; Zhu, H.; Hamer, R.M.; Styner, M.; Shen, D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb. Cortex 2012, 22, 2478–2485. [Google Scholar] [CrossRef]
- Howell, B.R.; Sanchez, M.M. Understanding behavioral effects of early life stress using the reactive scope and allostatic load models. Dev. Psychopathol. 2011, 23, 1001–1016. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, M.M.; Ladd, C.O.; Plotsky, P.M. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev. Psychopathol. 2001, 13, 419–449. [Google Scholar] [CrossRef]
- Blohm, G.M.; Lednicky, J.A.; Márquez, M.; White, S.K.; Loeb, J.C.; Pacheco, C.A.; Nolan, D.J.; Paisie, T.; Salemi, M.; Rodríguez-Morales, A.J.; et al. Evidence for Mother-to-Child Transmission of Zika Virus Through Breast Milk. Clin. Infect. Dis. 2018, 66, 1120–1121. [Google Scholar] [CrossRef] [Green Version]
- Brito, C.A.; Brito, C.C.; Oliveira, A.C.; Rocha, M.; Atanásio, C.; Asfora, C.; Matos, J.D.; Lima, A.S.; Albuquerque, M.F. Zika in Pernambuco: Rewriting the first outbreak. Rev. Soc. Bras. Med. Trop. 2016, 49, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Hall, V.; Walker, W.L.; Lindsey, N.P.; Lehman, J.A.; Kolsin, J.; Landry, K.; Rabe, I.B.; Hills, S.L.; Fischer, M.; Staples, J.E.; et al. Update: Noncongenital Zika Virus Disease Cases—50 U.S. States and the District of Columbia, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, O.; Beltrán, M.; Nelson, C.A.; Valencia, D.; Tolosa, N.; Farr, S.L.; Padilla, A.V.; Tong, V.T.; Cuevas, E.L.; Espinosa-Bode, A.; et al. Zika Virus Disease in Colombia—Preliminary Report. N. Engl. J. Med. 2016, 383, 44. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.B.; Dziuban, E.J.; Powell, K.; Bitsko, R.H.; Langley, G.; Lindsey, N.; Franks, J.L.; Russell, K.; Dasgupta, S.; Barfield, W.D.; et al. Characteristics of Children Aged <18 Years with Zika Virus Disease Acquired Postnatally—U.S. States, January 2015–July 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1082–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Z.J.M.; Hapuarachchi, H.P.; Barkham, T.; Chow, A.; Ng, L.C.; Lee, J.M.V.; Leo, Y.S.; Prem, K.; Lim, Y.H.G.; de Sessions, P.F.; et al. Outbreak of Zika virus infection in Singapore: An epidemiological, entomological, virological, and clinical analysis. Lancet Infect. Dis. 2017, 17, 813–821. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, N.P.; Porse, C.C.; Potts, E.; Hyun, J.; Sandhu, K.; Schiffman, E.; Cervantes, K.B.; White, J.L.; Mason, K.; Owens, K.; et al. Zika Virus Disease Enhanced Surveillance Working Group. Postnatally Acquired Zika Virus Disease Among Children, United States, 2016–2017. Clin. Infect. Dis. 2020, 70, 227–231. [Google Scholar] [CrossRef]
- Ramond, A.; Lobkowicz, L.; Clemente, N.S.; Vaughan, A.; Turchi, M.D.; Wilder-Smith, A.; Brickley, E.B. Postnatal symptomatic Zika virus infections in children and adolescents: A systematic review. PLoS Negl. Trop. Dis. 2020, 14, 0008612. [Google Scholar] [CrossRef] [PubMed]
- Read, J.S.; Torres-Velasquez, B.; Lorenzi, O.; Rivera Sanchez, A.; Torres-Torres, S.; Rivera, L.V.; Capre-Franceschi, S.M.; Garcia-Gubern, C.; Munoz-Jordan, J.; Santiago, G.A.; et al. Symptomatic Zika Virus Infection in Infants, Children, and Adolescents Living in Puerto Rico. JAMA Pediatr. 2018, 172, 686–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, D.M.; Vega, R.; Rodríguez, J.A.; Niño, Á.; Rodríguez, R.; Ortiz, Á.; DeLaura, I.; Bosch, I.; Narváez, C.F. Clinical, laboratory and immune aspects of Zika virus-associated encephalitis in children. Int. J. Infect. Dis. 2020, 90, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Tolosa, N.; Tinker, S.C.; Pacheco, O.; Valencia, D.; Botero, D.S.; Tong, V.T.; Mercado, M.; Gilboa, S.M.; Gonzalez, M.; Nelson, C.A.; et al. Zika Virus Disease in Children in Colombia, August 2015 to May 2016. Paediatr. Perinat. Epidemiol. 2017, 31, 537–545. [Google Scholar] [CrossRef]
- Burger-Calderon, R.; Bustos Carrillo, F.; Gresh, L.; Ojeda, S.; Sanchez, N.; Plazaola, M.; Katzelnick, L.; Mercado, B.L.; Monterrey, J.C.; Elizondo, D.; et al. Age-dependent manifestations and case definitions of paediatric Zika: A prospective cohort study. Lancet Infect. Dis. 2020, 20, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Cano, M.; Esquivel, R. Infección por virus Zika en el Hospital del Niño “Dr José Renán Esquivel” (Panamá): Revisión de casos de desde su introducción en Latinoamérica. Pediátr. Panamá 2018, 47, 15–19. [Google Scholar]
- Arzuza-Ortega, L.; Polo, A.; Pérez-Tatis, G.; López-García, H.; Parra, E.; Pardo-Herrera, L.C.; Rico-Turca, A.M.; Villamil-Gómez, W.; Rodríguez-Morales, A.J. Fatal Sickle Cell Disease and Zika Virus Infection in Girl from Colombia. Emerg. Infect. Dis. 2016, 22, 925–927. [Google Scholar] [CrossRef]
- Azevedo, R.S.; Araujo, M.T.; Martins Filho, A.J.; Oliveira, C.S.; Nunes, B.T.; Cruz, A.C.; Nascimento, A.G.; Medeiros, R.C.; Caldas, C.A.; Araujo, F.C.; et al. Zika virus epidemic in Brazil. I. Fatal disease in adults: Clinical and laboratorial aspects. J. Clin. Virol. 2016, 85, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento-Ospina, A.; Vasquez-Serna, H.; Jimenez-Canizales, C.E.; Villamil-Gomez, W.E.; Rodriguez-Morales, A.J. Zika virus associated deaths in Colombia. Lancet Infect. Dis. 2016, 16, 523–524. [Google Scholar] [CrossRef] [Green Version]
- Lannuzel, A.; Fergé, J.L.; Lobjois, Q.; Signate, A.; Rozé, B.; Tressières, B.; Madec, Y.; Poullain, P.; Herrmann, C.; Najioullah, F.; et al. Long-term outcome in neuroZika: When biological diagnosis matters. Neurology 2019, 92, 2406–2420. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, O.; Newton, S.M.; Daza, M.; Cates, J.E.; Reales, J.A.M.; Burkel, V.K.; Mercado, M.; Godfred-Cato, S.; Gonzalez, M.; Anderson, K.N.; et al. Neurodevelopmental findings in children 20–30 months of age with postnatal Zika infection at 1–12 months of age, Colombia, September–November 2017. Paediatr. Perinat. Epidemiol. 2020. epub online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.K.; Swiney, B.S.; Williams, S.L.; Huffman, J.N.; Lucas, K.; Wang, S.H.; Kapral, K.M.; Li, A.; Dikranian, K.T. Zika Virus Infection in the Developing Mouse Produces Dramatically Different Neuropathology Dependent on Viral Strain. J. Neurosci. 2020, 40, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Nem de Oliveira Souza, I.; Frost, P.S.; França, J.V.; Nascimento-Viana, J.B.; Neris, R.L.S.; Freitas, L.; Pinheiro, D.J.L.L.; Nogueira, C.O.; Neves, G.; Chimelli, L.; et al. Acute and chronic neurological consequences of early-life Zika virus infection in mice. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Boothe, R.G.; Dobson, V.; Teller, D.Y. Postnatal development of vision in human and nonhuman primates. Annu. Rev. Neurosci. 1985, 8, 495–545. [Google Scholar] [CrossRef]
- Callaghan, B.L.; Sullivan, R.M.; Howell, B.; Tottenham, N. The international society for developmental psychobiology Sackler symposium: Early adversity and the maturation of emotion circuits--a cross-species analysis. Dev. Psychobiol. 2014, 56, 1635–1650. [Google Scholar] [CrossRef] [Green Version]
- Mavigner, M.; Raper, J.; Kovacs-Balint, Z.; Gumber, S.; O’Neal, J.T.; Bhaumik, S.K.; Zhang, X.; Habib, J.; Mattingly, C.; McDonald, C.E.; et al. Postnatal Zika virus infection causes persistent abnormalities in brain structure, function, and behavior in infant macaques. Sci. Transl. Med. 2018, 10, 06975. [Google Scholar] [CrossRef] [Green Version]
- Raper, J.; Kovacs-Balint, Z.; Mavigner, M.; Gumber, S.; Burke, M.W.; Habib, J.; Mattingly, C.; Fair, D.; Earl, E.; Feczko, E.; et al. Long-term alterations in brain and behavior after postnatal Zika virus infections in infant macaques. Nat. Commun. 2020, 11, 1–12. [Google Scholar]
- Connolly, K.J.; Forssberg, H. Neurophysiology and Neuropsychology of Motor Development; Mac Keith Press: London, UK, 1997. [Google Scholar]
- Kalin, N.; Shelton, S.E.; Takahashi, L.K. Defensive behaviors in infant rhesus monkeys: Ontogeny and context dependent selective expression. Child. Dev. 1991, 62, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Balint, Z.; Feczko, E.; Pincus, M.J.; Howell, B.; Morin, E.; Earl, E.; Li, L.; Steele, J.; Styner, M.; Bachevalier, J.; et al. Early developmental trajectories of functional connectivity along the visual pathways in rhesus monkeys. Cereb. Cortex 2019, 29, 3514–3526. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Payne, C.; Moss, S.; Jones, W.R.; Bachevalier, J. Early developmental changes in visual social engagement in infant rhesus monkeys. Dev. Cogn. Neurosci. 2020, 43, 1–10. [Google Scholar] [CrossRef]
- Karere, G.M.; Kinnally, E.L.; Sanchez, J.N.; Famula, T.R.; Lyons, L.A.; Capitanio, J.P. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol. Psychiatry 2009, 65, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Raper, J.; Wilson, M.; Sanchez, M.; Payne, C.; Bachevalier, J. Increased anxiety-like behaviors, but blunted cortisol stress response after neonatal hippocampal lesions in monkeys. Psychoneuroendocrinology 2017, 76, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Raper, J.; Wilson, M.E.; Sanchez, M.; Machado, C.; Bachevalier, J. Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. Psychoneuroendocrinololgy 2013, 38, 1021–1035. [Google Scholar] [CrossRef] [Green Version]
- Rilling, J.K.; Winslow, J.T.; O’Brien, D.; Gutman, D.A.; Hoffman, J.M.; Kilts, C.D. Neural correlates of maternal separation in rhesus monkeys. Biol. Psychiatry 2001, 49, 146–157. [Google Scholar] [CrossRef]
- Sanchez, M.M.; Noble, P.M.; Lyon, C.K.; Plotsky, P.M.; Davis, M.; Nemeroff, C.B.; Winslow, J.T. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol. Psychiatry 2005, 57, 373–381. [Google Scholar] [CrossRef]
- Kagan, J.; Reznick, J.S.; Snidman, N. Biological bases of childhood shyness. Science 1988, 240, 167–171. [Google Scholar] [CrossRef]
- Kalin, N.; Shelton, S.E. Defensive behaviors in infant rhesus monkeys: Environmental cues and eurochemical regulation. Science 1989, 243, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Kalin, N.H. The Neurobiology of Fear. Sci. Am. 1993, 268, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Kalin, N.H.; Larson, C.; Shelton, S.E.; Davidson, R.J. Asymmetric Frontal Brain Activity, Cortisol, and Behavior Associated with Fearful Temperament in Rhesus Monkeys. Behav. Neurosci. 1998, 112, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Brito Ferreira, M.L.; Antunes de Brito, C.A.; Moreira, Á.J.P.; de Morais Machado, M.Í.; Henriques-Souza, A.; Cordeiro, M.T.; de Azevedo Marques, E.T.; Pena, L.J. Guillain-Barré Syndrome, Acute Disseminated Encephalomyelitis and Encephalitis Associated with Zika Virus Infection in Brazil: Detection of Viral RNA and Isolation of Virus during Late Infection. Am. J. Trop. Med. Hyg. 2017, 97, 1405–1409. [Google Scholar] [CrossRef] [Green Version]
- Cleto, T.L.; de Araújo, L.F.; Capuano, K.G.; Rego Ramos, A.; Prata-Barbosa, A. Peripheral Neuropathy Associated with Zika Virus Infection. Pediatr. Neurol. 2016, 65, 1–2. [Google Scholar] [CrossRef]
- Landais, A.; Césaire, A.; Fernandez, M.; Breurec, S.; Herrmann, C.; Delion, F.; Desprez, P. ZIKA vasculitis: A new cause of stroke in children? J. Neurol. Sci. 2017, 383, 211–213. [Google Scholar] [CrossRef]
- Marinho, P.E.S.; Alvarenga, P.P.M.; Lima, M.T.; de Souza Andrade, A.; Candiani, T.M.S.; Crispim, A.P.C.; Gasparini, M.C.S.; Castro, F.S.; de Sousa, A.Z.A.; Viegas, E.C.C.; et al. Central and peripheral nervous system involvement in Zika virus infection in a child. J. Neurovirol. 2019, 25, 893–896. [Google Scholar] [CrossRef]
- Mécharles, S.; Herrmann, C.; Poullain, P.; Tran, T.H.; Deschamps, N.; Mathon, G.; Landais, A.; Breurec, S.; Lannuzel, A. Acute myelitis due to Zika virus infection. Lancet 2016, 387, 1481. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, M.C.; Murphy, K.L.; Baxter, M.G. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br. J. Anaesth. 2017, 119, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Blue, S.N.; Kazama, A.M.; Bachevalier, J. Development of memory for spatial locations and object/place associations in infant rhesus macaques with and without neonatal hippocampal lesions. J. Int. Neuropsychol. Soc. 2013, 19, 1053–1064. [Google Scholar] [CrossRef] [Green Version]
- Dettmer, A.M.; Murphy, A.M.; Guitarra, D.; Slonecker, E.; Suomi, S.J.; Rosenberg, K.L.; Novak, M.A.; Meyer, J.S.; Hinde, K. Cortisol in Neonatal Mother’s Milk Predicts Later Infant Social and Cognitive Functioning in Rhesus Monkeys. Child Dev. 2018, 89, 525–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeamer, A.; Heuer, E.; Bachevalier, J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J. Neurosci. 2010, 30, 9157–9165. [Google Scholar] [CrossRef] [PubMed]
- Bachevalier, J.; Vargha-Khadem, F. The primate hippocampus: Ontogeny, early insult and memory. Curr. Opin. Neurobiol. 2005, 15, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Bachevalier, J. Nonhuman primate models of hippocampal development and dysfunction. Proc. Natl. Acad. Sci. USA 2019, 23, 26210–26216. [Google Scholar] [CrossRef] [Green Version]
- Snyder-Keller, A.; Kramer, L.D.; Zink, S.; Bolivar, V.J. Mouse Strain and Sex-Dependent Differences in Long-term Behavioral Abnormalities and Neuropathologies after Developmental Zika Infection. J. Neurosci. 2019, 39, 5393–5403. [Google Scholar] [CrossRef] [Green Version]
- Lanciotti, R.; Lambert, A.; Holodniy, M.; Saavedra, S.; del Carmen Castillo Signor, L. Phylogeny of Zika virus in Western Hemisphere, 2015. Emerg. Infect. Dis. 2016, 22, 933–935. [Google Scholar] [CrossRef]
- Carryl, H.; Van Rompay, K.K.; De Paris, K.; Burke, M.W. Hippocampal Neuronal Loss in Infant Macaques Orally Infected with Virulent Simian Immunodeficiency Virus (SIV). Brain Sci. 2017, 7, 40. [Google Scholar] [CrossRef]
- Cooper, E.R.; Hanson, C.; Diaz, C.; Mendez, H.; Abboud, R.; Nugent, R.; Pitt, J.; Rich, K.; Rodriguez, E.M.; Smeriglio, V. Encephalopathy and progression of human immunodeficiency virus disease in a cohort of children with perinatally acquired human immunodeficiency virus infection. Women and Infants Transmission Study Group. J. Pediatr. 1998, 132, 808–812. [Google Scholar] [CrossRef]
- Epstein, L.G.; Sharer, L.R.; Goudsmit, J. Neurological and neuropathological features of human immunodeficiency virus infection in children. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1988, 23, 19–23. [Google Scholar] [CrossRef]
- Hamprecht, K.; Goelz, R. Postnatal Cytomegalovirus Infection Through Human Milk in Preterm Infants: Transmission, Clinical Presentation, and Prevention. Clin. Perinatol. 2017, 44, 121–130. [Google Scholar] [CrossRef]
- Maness, N.J.; Schouest, B.; Singapuri, A.; Dennis, M.; Gilbert, M.H.; Bohm, R.P.; Schiro, F.; Aye, P.P.; Baker, K.; Van Rompay, K.K.A.; et al. Postnatal Zika virus infection of nonhuman primate infants born to mothers infected with homologous Brazilian Zika virus. Sci. Rep. 2019, 9, 12802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vannella, K.M.; Stein, S.; Connelly, M.; Swerczek, J.; Amaro-Carambot, E.; Coyle, E.M.; Babyak, A.; Winkler, C.W.; Saturday, G.; Gai, N.D.; et al. Nonhuman primates exposed to Zika virus in utero are not protected against reinfection at 1 year postpartum. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.R.; Razo, E.; Mitzey, A.; Newman, C.M.; Dudley, D.M.; Breitbach, M.E.; Semler, M.R.; Stewart, L.M.; Weiler, A.M.; Rybarczyk, S.; et al. Quantitative definition of neurobehavior, vision, hearing and brain volumes in macaques congenitally exposed to Zika virus. PLoS ONE 2020, 15, 0235877. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raper, J.; Chahroudi, A. Clinical and Preclinical Evidence for Adverse Neurodevelopment after Postnatal Zika Virus Infection. Trop. Med. Infect. Dis. 2021, 6, 10. https://doi.org/10.3390/tropicalmed6010010
Raper J, Chahroudi A. Clinical and Preclinical Evidence for Adverse Neurodevelopment after Postnatal Zika Virus Infection. Tropical Medicine and Infectious Disease. 2021; 6(1):10. https://doi.org/10.3390/tropicalmed6010010
Chicago/Turabian StyleRaper, Jessica, and Ann Chahroudi. 2021. "Clinical and Preclinical Evidence for Adverse Neurodevelopment after Postnatal Zika Virus Infection" Tropical Medicine and Infectious Disease 6, no. 1: 10. https://doi.org/10.3390/tropicalmed6010010
APA StyleRaper, J., & Chahroudi, A. (2021). Clinical and Preclinical Evidence for Adverse Neurodevelopment after Postnatal Zika Virus Infection. Tropical Medicine and Infectious Disease, 6(1), 10. https://doi.org/10.3390/tropicalmed6010010