Improved Electrochemical Properties of an Ni-Based YSZ Cermet Anode for the Direct Supply of Methane by Co Alloying with an Impregnation Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Phase Identification
3.2. Microstructure of the Anode
3.3. Cell Performance for a H2 Supply
3.4. Cell Performance for a CH4 Supply
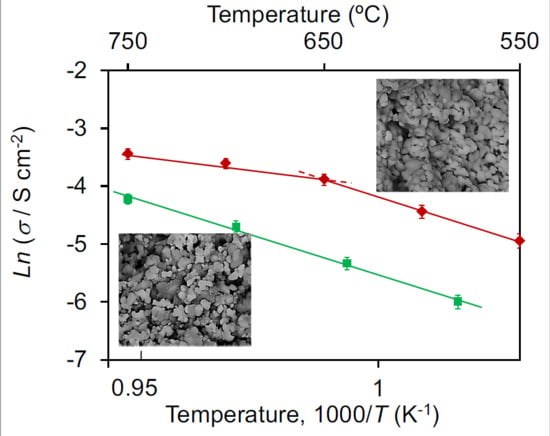
3.5. Temperature Dependence of the Anode Interface Conductivity
3.6. Prolonged Stability in CH4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkinson, A.; Barnett, S.A.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nat. Mater. 2004, 3, 17–24. [Google Scholar] [CrossRef]
- Zhan, Z.; Barnett, S.A. An octane-fueled solid oxide fuel cell. Science 2005, 308, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Vohs, J.M.; Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature 2000, 404, 265–267. [Google Scholar] [CrossRef]
- Liu, J.; Barnett, S.A. Operation of anode-supported solid oxide fuel cells on methane and natural gas. Solid State Ion. 2003, 158, 11–16. [Google Scholar] [CrossRef]
- Armstrong, E.N.; Park, J.; Minh, N.Q. High-performance direct solid oxide fuel cells. Electrochem. Solid-State Lett. 2012, 15, B75–B77. [Google Scholar] [CrossRef]
- Muccillo, R.; Muccillo, E.N.S.; Fonseca, F.C.; de Florio, D.Z. Characteristics and performance of electrolyte-supported solid oxide fuel cells under ethanol and hydrogen. J. Electrochem. Soc. 2008, 155, B232–B235. [Google Scholar] [CrossRef]
- Kaparaju, P.; Buendia, I.; Ellegaard, L.; Angelidakia, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.H.; Lee, D.J. Production of hydrogen and methane from wastewater sludge using anaerobic fermentation. Int. J. Hydrog. Energy 2007, 32, 677–682. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Manios, T. Enhanced methane and hydrogen production from municipal solid waste and agro-industrial by-products co-digested with crude glycerol. Bioresour. Technol. 2009, 100, 3043–3047. [Google Scholar] [CrossRef]
- Sanphoti, N.; Towprayoon, S.; Chaiprasert, P.; Nopharatana, A. The effects of leachate recirculation with supplemental water addition on methane production and waste decomposition in a simulated tropical landfill. J. Environ. Manag. 2006, 81, 27–35. [Google Scholar] [CrossRef]
- Gorte, R.J.; Vohs, J.M. Novel SOFC anodes for the direct electrochemical oxidation of hydrocarbons. J. Catal. 2003, 216, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Teraoka, Y. Equilibria in fuel cell gases I. Equilibrium compositions and reforming conditions. J. Electrochem. Soc. 2003, 150, A878–A884. [Google Scholar] [CrossRef]
- Mogensen, D.; Grunwaldt, J.D.; Hendriksen, P.V.; Dam-Johansen, K.; Nielsen, J.U. Internal steam reforming in solid oxide fuel cells: Status and opportunities of kinetic studies and their impact on modeling. J. Power Sources 2010, 196, 25–38. [Google Scholar] [CrossRef]
- Kim, H.; Lu, C.; Worrell, W.L.; Vohs, J.M.; Gorte, R.J. Cu-Ni cermet anodes for direct oxidation of methane in solid-oxide fuel cells. J. Electrochem. Soc. 2002, 149, A247–A250. [Google Scholar] [CrossRef]
- Sin, A.; Kopnin, E.; Dubitsky, Y.; Zaopo, A.; Arico, A.S.; Rosa, D.L.; Gullo, L.R.; Antonucci, V. Performance and life-time behavior of NiCu-CGO anodes for the direct electro-oxidation of methane in IT-SOFCs. J. Power Sources 2007, 164, 300–305. [Google Scholar] [CrossRef]
- Ringuedé, A.; Labrincha, J.A.; Frade, J.R. A combustion synthesis method to obtain alternative cermet materials for SOFC anodes. Solid State Ion. 2001, 141–142, 549–557. [Google Scholar] [CrossRef]
- McIntosh, S.; Vohs, J.M.; Gorte, R.J. Hydrocarbon deposits in the enhanced performance of direct-oxidation SOFCs. J. Electrochem. Soc. 2003, 150, A470–A476. [Google Scholar] [CrossRef]
- Jung, S.; Lu, C.; He, H.P.; Ahn, K.; Gorte, R.J.; Vohs, J.M. Influence of composition and Cu impregnation method on the performance of Cu/CeO2/YSZ SOFC anodes. J. Power Sources 2006, 154, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Hill, J.M. Carbon tolerance, electrochemical performance and stability of solid oxide fuel cells with Ni/yttria stabilized zirconia anodes impregnated with Sn and operated with methane. J. Power Sources 2012, 214, 185–194. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Ringuedé, A.; Bronine, D.; Frade, J.R. Ni1-xCox/YSZ cermet anodes for solid oxide fuel cells. Electrochem. Acta 2002, 48, 437–442. [Google Scholar] [CrossRef]
- Grgicak, C.M.; Pakulska, M.M.; O’Brien, J.S.; Giorgi, J.B. Synergistic effects of Ni1-xCox-YSZ and Ni1-xCux-YSZ alloyed cermet SOFC anodes for oxidation of hydrogen and methane fuels containing H2S. J. Power Sources 2008, 183, 26–33. [Google Scholar] [CrossRef]
- Cho, C.K.; Choi, B.H.; Lee, K.T. Effect of Co alloying on the electrochemical performance of Ni-Ce0.8Gd0.2O1.9 anodes for hydrocarbon-fueled solid oxide fuel cells. J. Alloys Compd. 2012, 541, 433–439. [Google Scholar] [CrossRef]
- Bošković, S.; Stevanović, M. Sintering of cobalt-doped nickel oxide. J. Mater. Sci. 1975, 10, 25–31. [Google Scholar] [CrossRef]
- Silva, G.C.; Muccillo, E.N.S. Electrical conductivity of yttria-stabilized zirconia with cobalt addition. Solid State Ion. 2009, 180, 835–838. [Google Scholar] [CrossRef]
- Venkataramanan, N.S.; Suvitha, A.; Mizuseki, H.; Kawazoe, Y. A theoretical study of the effects of transition metal dopants on the adsorption and dissociation of hydrogen on nickel clusters. Int. J. Quant. Chem. 2013, 113, 1940–1948. [Google Scholar] [CrossRef]
- Li, K.; Jiao, M.; Wang, Y.; Wu, Z. CH4 dissociation on NiM(111) (M = Co, Rh, Ir) surface: A first-principles study. Surf. Sci. 2013, 617, 149–155. [Google Scholar] [CrossRef]
- Liu, H.; Wang, B.; Fan, M.; Henson, N.; Zhang, Y.; Towler, B.F.; Harris, H.G. Study on carbon deposition associated with catalytic CH4 reforming by using density functional theory. Fuel 2013, 113, 712–718. [Google Scholar] [CrossRef]
- Weerapakkaroon, W.; Ogasa, K.; Sato, K. Electrochemical activity of Ni1-XCoX-Based cermet anode for methane oxidation. ECS Trans. 2007, 7, 1695–1699. [Google Scholar]
- Liu, C.; Cundari, T.R.; Wilson, A.K. CO2 reduction on transition metal (Fe, Co, Ni, and Cu) surfaces: In comparison with homogeneous catalysis. J. Phys. C. 2012, 116, 5681–5688. [Google Scholar] [CrossRef]





 : 0 h,
: 0 h,  : 60 h).
: 60 h).
 : 0 h,
: 0 h,  : 60 h).
: 60 h).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongsawatgul, N.; Chaianansutcharit, S.; Yamamoto, K.; Nanko, M.; Sato, K. Improved Electrochemical Properties of an Ni-Based YSZ Cermet Anode for the Direct Supply of Methane by Co Alloying with an Impregnation Method. Ceramics 2020, 3, 114-126. https://doi.org/10.3390/ceramics3010012
Wongsawatgul N, Chaianansutcharit S, Yamamoto K, Nanko M, Sato K. Improved Electrochemical Properties of an Ni-Based YSZ Cermet Anode for the Direct Supply of Methane by Co Alloying with an Impregnation Method. Ceramics. 2020; 3(1):114-126. https://doi.org/10.3390/ceramics3010012
Chicago/Turabian StyleWongsawatgul, Nicharee, Soamwadee Chaianansutcharit, Kazuhiro Yamamoto, Makoto Nanko, and Kazunori Sato. 2020. "Improved Electrochemical Properties of an Ni-Based YSZ Cermet Anode for the Direct Supply of Methane by Co Alloying with an Impregnation Method" Ceramics 3, no. 1: 114-126. https://doi.org/10.3390/ceramics3010012
APA StyleWongsawatgul, N., Chaianansutcharit, S., Yamamoto, K., Nanko, M., & Sato, K. (2020). Improved Electrochemical Properties of an Ni-Based YSZ Cermet Anode for the Direct Supply of Methane by Co Alloying with an Impregnation Method. Ceramics, 3(1), 114-126. https://doi.org/10.3390/ceramics3010012