Investigating the Potential Impact of Louisiana Coastal Restoration on the Trace Metal Geochemistry of Constructed Marshlands
Abstract
:1. Introduction
2. Study Site
3. Materials and Methods
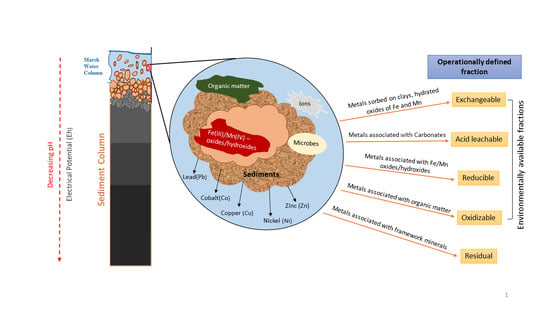
3.1. Sample Collection
3.2. Leaching Procedures and Reagents
3.3. Total Digestion of Sediment Samples
3.4. Analytical Methods
4. Results
5. Discussion
5.1. Comparison with Previous Studies
5.2. Fate of LMR Sediment-Associated Metals in New Coastal Marshlands
5.3. Estimating Pore Water Trace Metal Concentrations in Newly Constructed Marshlands
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Metal | Sample No. | Fraction 1 (%) | Fraction 2 (%) | Fraction 3 (%) | Fraction 4 (%) | Fraction 5 (%) |
|---|---|---|---|---|---|---|
| Pb | TU 171 | 0.12 a | 2.23 a | 8.77 ± 2.63 | 14.0 a | 74.9 ± 3.00 |
| TU 169 | b | 2.70 ± 0.08 | 6.73 ± 0.29 | 8.19 ± 0.81 | 82.4 ± 3.21 | |
| MRSS | 0.06 a | 7.64 ± 0.21 | 64.2 ± 1.09 | 11.9 ± 0.34 | 16.3 ± 1.40 | |
| MRBS | 0.02 a | 7.72 ± 0.65 | 33.2 ± 0.22 | 9.34 a | 49.7 ± 0.38 | |
| Zn | TU 171 | 0.24 a | 2.74 ± 0.06 | 5.21 ± 0.33 | 14.4 ± 1.44 | 77.5 ± 3.49 |
| TU 169 | 0.15 a | 1.48 ± 0.13 | 6.53 ± 0.59 | 16.1 ± 1.22 | 75.8 ± 2.05 | |
| MRSS | 1.79 ± 0.01 | 5.05 ± 0.18 | 33.4 ± 0.73 | 6.97 ± 0.21 | 52.8 ± 1.16 | |
| MRBS | b | 3.62 ± 0.09 | 22.8 ± 0.23 | 5.75 ± 0.23 | 67.1 ± 0.47 | |
| Co | TU 171 | 0.69 a | 3.96 a | 9.09 ± 0.51 | 23.7 ± 0.52 | 62.7 ± 1.45 |
| TU 169 | 0.57 a | 3.03 ± 0.34 | 11.3 ± 0.69 | 23.0 ± 2.17 | 62.1 ± 2.48 | |
| MRSS | 0.13 a | 2.25 a | 49.4 ± 1.82 | 7.38 ± 0.21 | 40.8 ± 1.95 | |
| MRBS | 0.03 a | 0.33 a | 42.5 ± 0.85 | 11.8 ± 0.21 | 45.3 ± 1.27 | |
| Ni | TU 171 | 0.24 a | 2.36 a | 9.27 ± 0.56 | 24.0 ± 0.84 | 64.1 ± 2.24 |
| TU 169 | 0.20 a | 2.12 ± 0.08 | 9.7 ± 0.46 | 23.1 ± 1.70 | 64.9 ± 1.16 | |
| MRSS | 0.48 a | 1.33 ± 0.07 | 17.7 ± 0.42 | 7.43 ± 0.25 | 73.1 ± 1.02 | |
| MRBS | b | 0.76 a | 22.4 ± 0.54 | 10.3 ± 0.27 | 66.5 ± 2.26 | |
| Cu | TU 171 | 0.59 a | 3.27 a | 3.68 a | 20.1 ± 1.09 | 66.3 ± 3.11 |
| TU 169 | 0.43 a | 2.88 ± 0.39 | 5.07 ± 0.39 | 19.7 ± 0.81 | 69.0 ± 4.62 | |
| MRSS | 0.70 ± 0.02 | 6.90 ± 0.13 | 30.7 ± 0.44 | 16.8 ± 0.17 | 42.9 ± 1.97 | |
| MRBS | 0.18 a | 0.99 ± 0.03 | 3.69 ± 0.05 | 9.40 ± 0.23 | 85.7 ± 1.88 |

| (A) Pre-dissolution | ||
| Mineral | 001 Peak Area | Relative Percent |
| Quartz | 49,440 | 82.95441199 |
| Microcline | 7571 | 12.70323328 |
| Albite | 2588 | 4.342354737 |
| Mica/Clay | Trace | |
| 59,599 | ||
| (B) Post-dissolution | ||
| Mineral | 001 Peak Area | Relative Percent |
| Quartz | 39,595 | 77.12760777 |
| Microcline | 11,742 | 22.87239223 |
| Mica/clay | Trace | |
| 51,337 | ||

References
- Day, J.W.; Templet, P.H. Consequences of sea level rise: Implications from the Mississippi delta. Coast. Manag. 1989, 17, 241–257. [Google Scholar] [CrossRef]
- Stanley, D.J.; Warne, A.G. Nile Delta: Recent Geological Evolution and Human Impact. Science 1993, 260, 628–634. [Google Scholar] [CrossRef] [PubMed]
- McInnes, K.L.; Walsh, K.J.E.; Hubbert, G.D.; Beer, T. Impact of Sea-level Rise and Storm Surges on a Coastal Community. Nat. Hazards 2003, 30, 187–207. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Lowe, J.A. Benefits of mitigation of climate change for coastal areas. Glob. Environ. Chang. 2004, 14, 229–244. [Google Scholar] [CrossRef]
- Curtis, K.J.; Schneider, A. Understanding the demographic implications of climate change: Estimates of localized population predictions under future scenarios of sea-level rise. Popul. Environ. 2011, 33, 28–54. [Google Scholar] [CrossRef]
- Woodruff, J.D.; Irish, J.L.; Camargo, S.J. Coastal flooding by tropical cyclones and sea-level rise. Nature 2013, 504, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giosan, L.; Syvitski, J.; Constantinescu, S.; Day, J.W. Climate change: Protect the world’s deltas. Nature 2014, 516, 31–33. [Google Scholar] [CrossRef] [Green Version]
- Nienhuis, P.H.; Leuven, R.S.E.W. River restoration and flood protection: Controversy or synergism. Hydrobiologia 2001, 444, 85–99. [Google Scholar] [CrossRef]
- Day, J.W.; Barras, J.; Clairain, E.; Johnston, J.; Justic, D.; Kemp, G.P.; Ko, J.; Lane, R.; Mitsch, W.J.; Steyer, G.; et al. Implications of global climatic change and energy cost and availability for the restoration of the Mississippi delta. Ecol. Eng. 2005, 24, 253–265. [Google Scholar] [CrossRef]
- Day, J.W.; Lane, R.R.; D’Elia, C.F.; Wiegman, A.R.H.; Rutherford, J.S.; Shaffer, G.P.; Brantley, C.G.; Kemp, G.P. Large Infrequently Operated River Diversions for Mississippi Delta Restoration. In Missississippi Delta Restoration; Estuaries of the World; Day, J., Erdman, J., Eds.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2018; pp. 113–133. [Google Scholar] [CrossRef]
- Bijker, W.E. American and Dutch coastal engineering: Differences in risk conception and differences in technological culture. Soc. Stud. Sci. 2007, 37, 143–151. [Google Scholar] [CrossRef]
- Allison, M.A.; Meselhe, E.A. The use of large water and sediment diversions in the lower Mississippi River (Louisiana) for coastal restoration. J. Hydrol. 2010, 387, 346–360. [Google Scholar] [CrossRef]
- Fabre, J.B. Sediment flux & fate for a large-scale diversion: The 2011 Mississippi River Flood, the Bonnet Carré Spillway, and the Implications for coastal Restoration in South Louisiana. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, LSU Digital Commons, Baton Rouge, LA, USA, 2012. Available online: https://pdfs.semanticscholar.org/9739/391e0d3863fb5f5db3586308412b5b3a94db.pdf (accessed on 26 May 2020).
- Jonkman, S.N.; Hillen, M.M.; Nicholls, R.J.; Kanning, W.; van Ledden, M. Costs of Adapting Coastal Defences to Sea-Level Rise—New Estimates and Their Implications. J. Coast. Res. 2013, 29, 1212–1226. [Google Scholar] [CrossRef]
- Temmerman, S.; Meire, P.; Bouma, T.J.; Herman, P.M.J.; Ysebaert, T.; De Vriend, H.J. Ecosystem-based coastal defence in the face of global change. Nature 2013, 504, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Stive, M.J.F.; De Schipper, M.A.; Luijendijk, A.P.; Aarninkhof, S.G.J.; Van Gelder-Maas, C.; Van Thiel de Vries, J.S.M.; De Vries, S.; Henriquez, M.; Marx, S.; Ranasinghe, R. A New Alternative to Saving Our Beaches from Sea-Level Rise: The Sand Engine. J. Coast. Res. 2013, 29, 1001–1008. [Google Scholar] [CrossRef]
- Chan, F.; Huq, H.; Pinter, N.; Zegwaard, A. Trends in flood risk management in deltas around the world: Are we going ‘soft’. Int. J. Water Gov. 2015, 3, 4. [Google Scholar]
- Olea, R.A.; Coleman, J.L., Jr. A Synoptic Examination of Causes of Land Loss in Southern Louisiana as Related to the Exploitation of Subsurface Geologic Resources. J. Coast. Res. 2014, 30, 1025–1044. [Google Scholar] [CrossRef]
- Törnqvist, T.E.; Jankowski, K.L.; Li, Y.; González, J.L. Tipping points of Mississippi Delta marshes due to accelerated sea-level rise. Sci. Adv. 2020, 6, eaaz5512. [Google Scholar] [CrossRef]
- Chamberlain, E.L.; Törnqvist, T.E.; Shen, Z.; Mauz, B.; Wallinga, J. Anatomy of Mississippi Delta growth and its implications for coastal restoration. Sci. Adv. 2018, 4, eaar4740. [Google Scholar] [CrossRef] [Green Version]
- CPRA. Coastal Restoration Authority of Louisiana. Louisiana’s comprehensive master plan for a sustainable coast. Coast. Prot. Restor. Auth. Baton Rouge 2012, 188–190. Available online: http://www.coastalmasterplan.la.gov (accessed on 19 November 2019).
- CPRA. Coastal Restoration Authority of Louisiana. Louisiana’s comprehensive master plan for a sustainable coast. Appendix B: People and The Landscape. 2017. Available online: http://coastal.la.gov/wp-content/uploads/2017/04/Appendix-B_People-and-theLandscape_FINAL.pdf (accessed on 16 May 2020).
- Rodning, C.B.; Mehta, J.M. Resilience and Persistent Places in the Mississippi River Delta of Southeastern Louisiana; Center for Archealogical Investigations. In Occassional Paper; SIU Press: Carbondale, IL, USA, 2015; p. 42. [Google Scholar]
- Kolker, A.S.; Cable, J.E.; Johannesson, K.H.; Allison, M.A.; Inniss, L.V. Pathways and processes associated with the transport of groundwater in deltaic systems. J. Hydrol. 2013, 498, 319–334. [Google Scholar] [CrossRef]
- Allison, M.A.; Demas, C.R.; Ebersole, B.A.; Kleiss, B.A.; Little, C.D.; Meselhe, E.A.; Powell, N.J.; Pratt, T.C.; Vosburg, B.M. A water and sediment budget for the lower Mississippi–Atchafalaya River in flood years 2008–2010: Implications for sediment discharge to the oceans and coastal restoration in Louisiana. J. Hydrol. 2012, 432–433, 84–97. [Google Scholar] [CrossRef]
- Day, J.W.; Moerschbaecher, M.; Pimentel, D.; Hall, C.; Yáñez-Arancibia, A. Sustainability and place: How emerging mega-trends of the 21st century will affect humans and nature at the landscape level. Ecol. Eng. 2014, 65, 33–48. [Google Scholar] [CrossRef]
- Amer, R.; Kolker, A.S.; Muscietta, A. Propensity for erosion and deposition in a deltaic wetland complex: Implications for river management and coastal restoration. Remote Sens. Environ. 2017, 199, 39–50. [Google Scholar] [CrossRef]
- Xu, K.; Bentley, S.J.; Day, J.W.; Freeman, A.M. A review of sediment diversion in the Mississippi River Deltaic Plain. Estuar. Coast. Shelf Sci. 2019, 225, 106241. [Google Scholar] [CrossRef]
- White, E.D.; Meselhe, E.; Reed, D.; Renfro, A.; Snider, N.P.; Wang, Y. Mitigating the Effects of Sea-Level Rise on Estuaries of the Mississippi Delta Plain Using River Diversions. Water 2019, 11, 2028. [Google Scholar] [CrossRef] [Green Version]
- USACE. United States Army Corps of Engineers. Mid-Barataria Sediment Diversion Project: Final Scoping Report. USACE Website. 2018. Available online: https://www.mvn.usace.army.mil/Portals/56/docs/regulatory/permits/EIS/2018_MBSD_Scoping%20Report.pdf (accessed on 8 June 2020).
- Cappuyns, V.; Swennen, R. Kinetics of element release during combined oxidation and pHstat leaching of anoxic river sediments. Appl. Geochem. 2005, 20, 1169–1179. [Google Scholar] [CrossRef]
- Davidson, G.R.; Bennett, S.J.; Beard, W.C.; Waldo, P. Trace Elements in Sediments of an Aging Reservoir in Rural Mississippi: Potential for Mobilization Following Dredging. Water Air Soil Pollut. 2005, 163, 281–292. [Google Scholar] [CrossRef]
- Miao, S.; De Laune, R.D.; Jugsujinda, A. Influence of sediment redox conditions on release/solubility of metals and nutrients in a Louisiana Mississippi River deltaic plain freshwater lake. Sci. Total Environ. 2006, 371, 334–343. [Google Scholar] [CrossRef]
- Trefry, J.H.; Metz, S.; Trocine, R.P.; Nelsen, T.A. A Decline in Lead Transport by the Mississippi River. Science 1985, 230, 439–441. [Google Scholar] [CrossRef]
- Corbett, R.D.; McKee, B.; Allison, M.A. Nature of decadal-scale sediment accumulation on the western shelf of the Mississippi River delta. Cont. Shelf Res. 2006, 26, 2125–2140. [Google Scholar] [CrossRef]
- Trefry, J.H.; Presley, B.J. Heavy metal transport from the Mississippi River to the Gulf of Mexico. In Marine Pollutant Transfer; Windom, H.L., Duce, R.A., Eds.; Heath and Co., Lexington Books: Lexington, MA, USA, 1976; pp. 39–76. [Google Scholar]
- Trefry, J.H.; Shokes, R.F. Chapter 4 History of Heavy-Metal Inputs to Mississippi Delta Sediments. In Elsevier Oceanography Series; Geyer, R.A., Ed.; Elsevier: Amsterdam, The Netherlands, 1981; Volume 27, pp. 193–208. [Google Scholar] [CrossRef]
- Trefry, J.H.; Presley, B.J. Heavy Metals in Sediments from San Antonio Bay and the Northwest Gulf of Mexico. Environ. Geol. 1976, 1, 283–294. [Google Scholar] [CrossRef]
- USEPA. US Environmental Protection Agency. EPA Takes Final Step in Phaseout of Leaded Gasoline. US Environmental Protection Agency Washington, DC: EPA’s Web Archive; 1996. Available online: https://archive.epa.gov/epa/aboutepa/epa-takes-final-step-phaseout-leadedgasoline.html (accessed on 11 June 2020).
- Mielke, H.W.; Anderson, J.C.; Berry, K.J.; Mielke, P.W., Jr.; Chaney, R.L.; Leech, M. Lead concentrations in inner-city soils as a factor in the child lead problem. Am. J. Public Health 1983, 73, 1366–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielke, H.W.; Dugas, D.; Mielke, P.W., Jr.; Smith, K.S.; Gonzales, C.R. Associations between soil lead and childhood blood lead in urban New Orleans and rural Lafourche Parish of Louisiana. Environ. Health Perspect. 1997, 105, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Mielke, H.W.; Gonzales, C.R.; Powell, E.; Jartun, M.; Mielke, P.W. Nonlinear association between soil lead and blood lead of children in metropolitan New Orleans, Louisiana: 2000–2005. Sci. Total Environ. 2007, 388, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Mielke, H.W.; Powell, E.T.; Gonzales, C.R.; Mielke, P.W. Potential lead on play surfaces: Evaluation of the “PLOPS” sampler as a new tool for primary lead prevention. Environ. Res. 2007, 103, 154–159. [Google Scholar] [CrossRef]
- Mielke, H.W.; Reagan, P.L. Soil is an important pathway of human lead exposure. Environ. Health Perspect. 1998, 106, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Laidlaw, M.A.S.; Filippelli, G.M. Resuspension of urban soils as a persistent source of lead poisoning in children: A review and new directions. Appl. Geochem. 2008, 23, 2021–2039. [Google Scholar] [CrossRef]
- Laidlaw, M.A.S.; Mielke, H.W.; Filippelli, G.M.; Johnson, D.L.; Gonzales, C.R. Seasonality and Children’s Blood Lead Levels: Developing a Predictive Model Using Climatic Variables and Blood Lead Data from Indianapolis, Indiana, Syracuse, New York, and New Orleans, Louisiana (USA). Environ. Health Perspect. 2005, 113, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.; Lin, Q.; Wiehe, S.; Liu, G.; Rosenman, M.; Fuller, T.; Wang, J.; Filippelli, G.M. Spatial relationships between lead sources and children’s blood lead levels in the urban center of Indianapolis (USA). Environ. Geochem. Health 2013, 35, 171–183. [Google Scholar] [CrossRef]
- Filippelli, G.M.; Adamic, J.; Nichols, D.; Shukle, J.; Frix, E. Mapping the Urban Lead Exposome: A Detailed Analysis of Soil Metal Concentrations at the Household Scale Using Citizen Science. Int. J. Env. Res. Public Health 2018, 15, 1531. [Google Scholar] [CrossRef] [Green Version]
- DeLaune, R.D.; Reddy, C.N.; Patrick, W.H. Accumulation of Plant Nutrients and Heavy Metals through Sedimentation Processes and Accretion in a Louisiana Salt Marsh. Estuaries 1981, 4, 328–334. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal uptake, transport and release by wetland plants: Implications for phytoremediation and restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Laws, E.A.; Gambrell, R. Trace element remobilization following the resuspension of sediments under controlled redox conditions: City Park Lake, Baton Rouge, LA. Appl. Geochem. 2013, 28, 91–99. [Google Scholar] [CrossRef]
- Michalec, B.K.; Lenart-Boroń, A.M.; Cupak, A.K.; Wałęga, A.S. The evaluation of heavy metal content in water and sediments of small reservoirs in light of various environmental quality regulations. J. Environ. Sci. Health Part A 2014, 49, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.T., III; Thompson, D.E. The Geology of Mississippi; University Press of Mississippi Jackson and Mississippi Department of Environmental Quality: Jackson, MS, USA, 2016; Volume 692, p. 751. [Google Scholar]
- Surbeck, C.Q.; Davidson, G.R.; Wren, D.G. Long-term metal and arsenic mobility between wetlands and lakes: Variable histories within the same floodplain. Appl. Geochem. 2018, 96, 244–251. [Google Scholar] [CrossRef]
- Howeler, R.H. The Oxygen Status of Lake Sediments. J. Environ. Qual. 1972, 1, 366–371. [Google Scholar] [CrossRef]
- Calmano, W.; Hong, J.; Förstner, U. Binding and Mobilization of Heavy Metals in Contaminated Sediments Affected by pH and Redox Potential. Water Sci. Technol. 1993, 28, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; A Wiley-Interseience Publication, John Wiley & Sons, INC: New York, NY, USA, 2012. [Google Scholar]
- Riba, I.; García-Luque, E.; Blasco, J.; DelValls, T.A. Bioavailability of heavy metals bound to estuarine sediments as a function of pH and salinity values. Chem. Speciat. Bioavailab. 2003, 15, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Wong, V.N.L.; Johnston, S.G.; Burton, E.D.; Bush, R.T.; Sullivan, L.A.; Slavich, P.G. Seawater-induced mobilization of trace metals from mackinawite-rich estuarine sediments. Water Res. 2013, 47, 821–832. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhao, P.; Chen, L.; Yan, C.; Yan, Y.; Chi, Q. Metal release from contaminated coastal sediments under changing pH conditions: Implications for metal mobilization in acidified oceans. Mar. Pollut. Bull. 2015, 101, 707–715. [Google Scholar] [CrossRef]
- Flowers, G.C.; Isphording, W.C. Heavy metal geochemistry of the Pontchartrain-Maurepas estuarine complex. Gulf Coast Association of Geological Societies and Gulf Coast Section of SEPM. Soc. Econ. AAPG Bulletin 1990, 74, 9. [Google Scholar] [CrossRef]
- Tang, J.; Whittecar, G.R.; Johannesson, K.H.; Daniels, W.L. Potential contaminants at a dredged spoil placement site, Charles City County, Virginia, as revealed by sequential extraction. Geochem. Trans. 2004, 5, 49. [Google Scholar] [CrossRef]
- Piper, D.Z.; Ludington, S.; Duval, J.S.; Taylor, H.E. Geochemistry of bed and suspended sediment in the Mississippi river system: Provenance versus weathering and winnowing. Sci. Total Environ. 2006, 362, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.A.; Andrews, J.E.; Jickells, T. Nitrous oxide and methane fluxes vs. carbon, nitrogen and phosphorous burial in new intertidal and saltmarsh sediments. Sci. Total Environ. 2012, 434, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Mohajerin, T.J.; Helz, G.R.; Johannesson, K.H. Tungsten–molybdenum fractionation in estuarine environments. Geochim. Cosmochim. Acta 2016, 177, 105–119. [Google Scholar] [CrossRef] [Green Version]
- Telfeyan, K.; Breaux, A.; Kim, J.; Cable, J.E.; Kolker, A.S.; Grimm, D.A.; Johannesson, K.H. Arsenic, vanadium, iron, and manganese biogeochemistry in a deltaic wetland, southern Louisiana, USA. Mar. Chem. 2017, 192, 32–48. [Google Scholar] [CrossRef]
- Telfeyan, K.; Breaux, A.; Kim, J.; Kolker, A.S.; Cable, J.E.; Johannesson, K.H. Cycling of oxyanion-forming trace elements in groundwaters from a freshwater deltaic marsh. Estuar. Coast. Shelf Sci. 2018, 204, 236–263. [Google Scholar] [CrossRef]
- Lane, R.R.; Day, J.W.; Thibodeaux, B. Water quality analysis of a freshwater diversion at Caernarvon, Louisiana. Estuaries 1999, 22, 327–336. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Day, J.W. Restoration of wetlands in the Mississippi–Ohio–Missouri (MOM) River Basin: Experience and needed research. Ecol. Eng. 2006, 26, 55–69. [Google Scholar] [CrossRef]
- Snedden, G.A.; Cable, J.E.; Swarzenski, C.; Swenson, E. Sediment discharge into a subsiding Louisiana deltaic estuary through a Mississippi River diversion. Estuar. Coast. Shelf Sci. 2007, 71, 181–193. [Google Scholar] [CrossRef]
- Kolker, A.S.; Miner, M.D.; Weathers, H.D. Depositional dynamics in a river diversion receiving basin: The case of the West Bay Mississippi River Diversion. Estuar. Coast. Shelf Sci. 2012, 106, 1–12. [Google Scholar] [CrossRef]
- Das, A.; Justic, D.; Inoue, M.; Hoda, A.; Huang, H.; Park, D. Impacts of Mississippi River diversions on salinity gradients in a deltaic Louisiana estuary: Ecological and management implications. Estuar. Coast. Shelf Sci. 2012, 111, 17–26. [Google Scholar] [CrossRef]
- Elsey-Quirk, T.; Graham, S.A.; Mendelssohn, I.A.; Snedden, G.; Day, J.W.; Twilley, R.R.; Shaffer, G.; Sharp, L.A.; Pahl, J.; Lane, R.R. Mississippi river sediment diversions and coastal wetland sustainability: Synthesis of responses to freshwater, sediment, and nutrient inputs. Estuar. Coast. Shelf Sci. 2019, 221, 170–183. [Google Scholar] [CrossRef]
- Bridgeman, J.G. Understanding Mississippi Delta subsidence through stratigraphic and geotechnical analysis of a continuous Holocene core at a subsidence superstation. Tulane University School of Science and Engineering. 2018. Available online: https://search.proquest.com/openview/c196964a9d9b3ba6ab76d51301b5c49c/1?pqorigsite=gscholar&cbl=18750&diss=y (accessed on 13 June 2020).
- Allision, M.; Tulane University, New Orleans, Louisiana, United Sates. Personal communication, 2019.
- Chamberlain, E.L.; Wallinga, J. Seeking enlightenment of fluvial sediment pathways by optically stimulated luminescence signal bleaching of river sediments and deltaic deposits. Earth Surf. Dyn. 2019, 7, 723–726. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.E.T. A Geochronological and Stratigraphic Reconstruction of the Middle Barataria Bay Receiving Basin. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, LSU Digital Commons, Baton Rouge, LA, USA, 2016. Available online: https://digitalcommons.lsu.edu/gradschool_theses/4427 (accessed on 26 May 2020).
- Bentley, S.J.; Xu, K.; Chen, Q. Data Report: Geological and Geotechnical Characterization for Lower Barataria Bay and Lower Breton Sound Diversion Receiving Basins; Coastal Studies Technical Report for the Water Institute of the Gulf; The Water Institute of the Gulf: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Bentley, S.J.; Xu, K.; Chen, Q. Geological and Geotechnical Characterization for Middle Barataria Bay and Middle Breton Sound Diversion Receiving Basins; Coastal Studies Technical Report for the Water Institute of the Gulf; The Water Institute of the Gulf: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Breaux, A.M. Utilization of Shallow Seismic, Resistivity Profiling, and Sediment Core Analyses for Identification of Semi-Permeable Sediments that Act as Conduits for Submarine Groundwater Discharge, Barataria Bay, Louisiana. Master’s Thesis, (10143955). Tulane University, New Orleans, LU, USA, 2015. Available online: https://search.proquest.com/openview/662a4af13ec39757fa0fc95e75c36eef/1?pqorigsite=gscholar&cbl=18750&diss=y (accessed on 20 May 2020).
- Meselhe, E.A.; Georgiou, I.; Allison, M.A.; McCorquodale, J.A. Numerical modeling of hydrodynamics and sediment transport in lower Mississippi at a proposed delta building diversion. J. Hydrol. 2013, 472–473, 340–354. [Google Scholar] [CrossRef]
- CPRA. Coastal Restoration Authority of Louisiana. Mid-Barataria and Mid-Breton Sediment Diversions: Overview and Frequently asked Questions; 2018. Available online: http://coastal.la.gov/wp-content/uploads/2018/03/OVERVIEW_FAQs_Mid-Barataria-and-Mid-Breton-Sediment-Diversions.pdf (accessed on 13 June 2020).
- USACE. United States Army Corps of Engineers. Environmental Impact Statement: Mid-Barataria Sediment Diversion. 2020. Available online: https://www.mvn.usace.army.mil/Missions/Regulatory/Permits/Mid-Barataria-Sediment-Diversion-EIS (accessed on 8 June 2020).
- CPRA. Coastal Restoration Authority of Louisiana. Mississippi River Mid-Basin Sediment Diversion Program: Timeline. 2020. Available online: http://coastal.la.gov/our-work/key-initiatives/%20diversion%20-program/timeline/ (accessed on 13 June 2020).
- Thorne, C.R.; Harmar, O.P.; Wallerstein, N. Sediment Transport in the Lower Mississippi River; Nottingham University (United Kingdom) Department of Geography: Nottingham, UK, 2000. [Google Scholar]
- Rosenheim, B.E.; Roe, K.M.; Roberts, B.J.; Kolker, A.S.; Allison, M.A.; Johannesson, K.H. River discharge influences on particulate organic carbon age structure in the Mississippi/Atchafalaya River System. Glob. Biogeochem. Cycles 2013, 27, 154–166. [Google Scholar] [CrossRef]
- Hijma, M.P.; Shen, Z.; Törnqvist, T.E.; Mauz, B. Late Holocene evolution of a coupled, mud-dominated delta plain–chenier plain system, coastal Louisiana, USA. Earth Surf. Dyn. 2017, 5, 689–710. [Google Scholar] [CrossRef] [Green Version]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Willis, S.S.; Johannesson, K.H. Controls on the geochemistry of rare earth elements in sediments and groundwaters of the Aquia aquifer, Maryland, USA. Chem. Geol. 2011, 285, 32–49. [Google Scholar] [CrossRef]
- Presley, B.J.; Trefry, J.H.; Shokes, R.F. Heavy metal inputs to Mississippi Delta sediments. Water Air Soil Pollut. 1980, 13, 481–494. [Google Scholar] [CrossRef]
- Swarzenski, P.W.; Baskaran, M.; Rosenbauer, R.J.; Orem, W.H. Historical trace element distribution in sediments from the Mississippi River delta. Estuaries Coasts 2006, 29, 1094–1107. [Google Scholar] [CrossRef]
- Garbarino, J.R.; Hayes, H.C.; Roth, D.A.; Antweiler, R.C.; Brinton, T.I.; Taylor, H.E. Heavy metals in the Mississippi River. Geol. Surv. Circ. 1996, 1133, 53–72. Available online: https://pubs.usgs.gov/circ/circ1133/heavy-metals.html (accessed on 21 January 2020).
- Filippelli, G.M.; Laidlaw, M.A.S.; Latimer, J.C.; Raftis, R. Urban lead poisoning and medical geology: An unfinished story. GSA Today 2005, 15, 4–11. [Google Scholar] [CrossRef]
- Fitzpatrick, M.L.; Long, D.T.; Pijanowski, B.C. Exploring the effects of urban and agricultural land use on surface water chemistry, across a regional watershed, using multivariate statistics. Appl. Geochem. 2007, 22, 1825–1840. [Google Scholar] [CrossRef]
- Huser, B.; Köhler, S.; Wilander, A.; Johansson, K.; Fölster, J. Temporal and spatial trends for trace metals in streams and rivers across Sweden (1996–2009). Biogeosciences 2011, 8, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
- Mielke, H.W.; Zahran, S. The urban rise and fall of air lead (Pb) and the latent surge and retreat of societal violence. Environ. Int. 2012, 43, 48–55. [Google Scholar] [CrossRef]
- Han, L.; Gao, B.; Hao, H.; Zhou, H.; Lu, J.; Sun, K. Lead contamination in sediments in the past 20 years: A challenge for China. Sci. Total Environ. 2018, 640–641, 746–756. [Google Scholar] [CrossRef]
- Stets, E.G.; Lee, C.J.; Lytle, D.A.; Schock, M.R. Increasing chloride in rivers of the conterminous U.S. and linkages to potential corrosivity and lead action level exceedances in drinking water. Sci. Total Environ. 2018, 613–614, 1498–1509. [Google Scholar] [CrossRef]
- Viers, J.; Dupré, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers: New insights from a new database. Sci. Total Environ. 2009, 407, 853–868. [Google Scholar] [CrossRef]
- Reiman, J.H.; Xu, Y.J.; He, S.; DelDuco, E.M. Metals geochemistry and mass export from the Mississippi-Atchafalaya River system to the Northern Gulf of Mexico. Chemosphere 2018, 205, 559–569. [Google Scholar] [CrossRef]
- Shiller, A.M. Dissolved trace elements in the Mississippi River: Seasonal, interannual, and decadal variability. Geochim. Cosmochim. Acta 1997, 61, 4321–4330. [Google Scholar] [CrossRef]
- Stolpe, B.; Guo, L.; Shiller, A.M.; Hassellöv, M. Size and composition of colloidal organic matter and trace elements in the Mississippi River, Pearl River and the northern Gulf of Mexico, as characterized by flow field-flow fractionation. Mar. Chem. 2010, 118, 119–128. [Google Scholar] [CrossRef]
- Davis, J.A.; Gloor, R. Adsorption of dissolved organics in lake water by aluminum oxide. Effect of molecular weight. Environ. Sci. Technol. 1981, 15, 1223–1229. [Google Scholar] [CrossRef]
- Vermeer, A.W.P.; Koopal, L.K. Adsorption of Humic Acids to Mineral Particles. 2. Polydispersity Effects with Polyelectrolyte Adsorption. Langmuir 1998, 14, 4210–4216. [Google Scholar] [CrossRef]
- Evanko, C.R.; Dzombak, D.A. Surface Complexation Modeling of Organic Acid Sorption to Goethite. J. Colloid Interface Sci. 1999, 214, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Filius, J.D.; Lumsdon, D.G.; Meeussen, J.C.L.; Hiemstra, T.; Van Riemsdijk, W.H. Adsorption of fulvic acid on goethite. Geochim. Cosmochim. Acta 2000, 64, 51–60. [Google Scholar] [CrossRef]
- Liang, L.; Luo, L.; Zhang, S. Adsorption and desorption of humic and fulvic acids on SiO2 particles at nano- and micro-scales. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 126–130. [Google Scholar] [CrossRef]
- Chotzen, R.A.; Polubesova, T.; Chefetz, B.; Mishael, Y.G. Adsorption of soil-derived humic acid by seven clay minerals: A systematic study. Clays Clay Miner. 2016, 64, 628–638. [Google Scholar] [CrossRef]
- Larocque, A.C.L.; Rasmussen, P.E. An overview of trace metals in the environment, from mobilization to remediation. Environ. Geol. 1998, 33, 85–91. [Google Scholar] [CrossRef]
- Meador, J. Rationale and Procedures for Using the Tissue-Residue Approach for Toxicity Assessment and Determination of Tissue, Water, and Sediment Quality Guidelines for Aquatic Organisms. Hum. Ecol. Risk Assess. Int. J. 2006, 12, 1018–1073. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.J.; Ali, A.; DeLaune, R.D. Heavy metal distribution and water quality characterization of water bodies in Louisiana’s Lake Pontchartrain Basin, USA. Environ. Monit. Assess. 2016, 188, 628. [Google Scholar] [CrossRef] [PubMed]
- Mirlean, N.; Ferraz, A.H.; Seus-Arrache, E.R.; Andrade, C.F.F.; Costa, L.P.; Johannesson, K.H. Mercury and selenium in the Brazilian subtropical marine products: Food composition and safety. J. Food Compos. Anal. 2019, 84, 103310. [Google Scholar] [CrossRef]
- Mitra, S.; Sudarshan, M.; Jonathan, M.P.; Sarkar, S.K.; Thakur, S. Spatial and seasonal distribution of multi-elements in suspended particulate matter (SPM) in tidally dominated Hooghly river estuary and their ecotoxicological relevance. Environ. Sci. Pollut. Res. 2020, 27, 12658–12672. [Google Scholar] [CrossRef] [PubMed]
- Quintana, G.; Mirlean, N.; Costa, L.P.; Johannesson, K. Mercury distributions in sediments of an estuary subject to anthropogenic hydrodynamic alterations (Patos Estuary, Southern Brazil). Environ. Monit. Assess. 2020, 192, 266. [Google Scholar] [CrossRef]
- Davranche, M.; Bollinger, J.; Bril, H. Effect of reductive conditions on metal mobility from wasteland solids: An example from the Mortagne-du-Nord site (France). Appl. Geochem. 2003, 18, 383–394. [Google Scholar] [CrossRef]
- Hering, J.G.; Kneebone, P.E. Biogeochemical controls on arsenic occurrence and mobility in water supplies. In Environmental Chemistry of Arsenic; Frankenberger, W.T., Ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 155–181. [Google Scholar]
- Johannesson, K.H.; Yang, N.; Trahan, A.S.; Telfeyan, K.; Jade Mohajerin, T.; Adebayo, S.B.; Akintomide, O.A.; Chevis, D.A.; Datta, S.; White, C.D. Biogeochemical and reactive transport modeling of arsenic in groundwaters from the Mississippi River delta plain: An analog for the As-affected aquifers of South and Southeast Asia. Geochim. Cosmochim. Acta 2019, 264, 245–272. [Google Scholar] [CrossRef]
- Osipov, V.I. Density of clay minerals. Soil Mech. Found. Eng. 2012, 48, 231–240. [Google Scholar] [CrossRef]
- USEPA. US Environmental Protection Agency. Edition of the Drinking Water Standards and Health Advisories; EPA's Web Archive, EPA 822-F-18–001; Office of Water; US Environmental Protection Agency: Washington, DC, USA, 2018; Available online: https://www.epa.gov/sites/production/files/2018–03/documents/dwtable2018.pdf (accessed on 6 July 2020).
- USEPA. US Environmental Protection Agency. Framework For Developing Suspended and Bedded Sediment (Sabs) Water Quality Criteria; EPA 822/R-06/001. U.S. EPA; Office of Water; Office of Research and Development: Washington, DC, USA, 2006; Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=164423 (accessed on 6 July 2020).
- Dallinger, R.; Prosi, F.; Segner, H.; Back, H. Contaminated food and uptake of heavy metals by fish: A review and a proposal for further research. Oecologia 1987, 73, 91–98. [Google Scholar] [CrossRef]
- Schmitt, C.J. Concentrations of Arsenic, Cadmium, Copper, Lead, Selenium, and Zinc in Fish from the Mississippi River Basin, 1995. Environ. Monit. Assess. 2004, 90, 289–321. [Google Scholar] [CrossRef]
- Rogowski, D.L.; Soucek, D.J.; Levengood, J.M.; Johnson, S.R.; Chick, J.H.; Dettmers, J.M.; Pegg, M.A.; Epifanio, J.M. Contaminant concentrations in Asian carps, invasive species in the Mississippi and Illinois Rivers. Environ. Monit. Assess. 2009, 157, 211–222. [Google Scholar] [CrossRef]


| Fraction | Reagent | Target Phase |
|---|---|---|
| 1 | 1 M CH3COONa; pH 8.2, 1 h shaking (25 °C) | TE weakly adsorbed on mineral surfaces |
| 2 | 1 M CH3COONa adjusted to pH 5 with CH3COOH: 5 h shaking (25 °C) | TE associated with carbonate minerals |
| 3 | 0.04 M NH2OH•HCl in 25% (v/v) CH3COOH; 6 h shaking (96 °C) | TE associated with reducible Fe/Mn oxides/oxyhydroxides |
| 4 | 0.02 M HNO3 + 30% H2O2 adjusted to pH 2 with HNO3; 5 h shaking (85 °C). Then 3.2 M CH3COONH4 in 20% HNO3; shaking for 30 min (25 °C) | TE associated with oxidizable sedimentary organic matter and some sulfide minerals |
| 5 | Concentrated HNO3 and HF (heated to near dryness and re-dissolved in 2% HNO3 | TE within the crystalline structure of silicate minerals |
| Metal | Sample No. | Fraction 1 (mg kg−1) | Fraction 2 (mg kg−1) | Fraction 3 (mg kg−1) | Fraction 4 (mg kg−1) | Fraction 5 (mg kg−1) | Sum of Fractions (mg kg−1) | Total Sediment Digestion (mg kg−1) | Recovery (%) |
|---|---|---|---|---|---|---|---|---|---|
| Pb | TU 171 | 0.01 a | 0.12 a | 0.48 ± 0.14 | 0.76 a | 4.1 ± 0.16 | 5.47 | 5.26 ± 0.15 | 104 |
| TU 169 | b | 0.17 ± 0.01 | 0.42 ± 0.02 | 0.51 ± 0.05 | 5.1 ± 0.20 | 6.19 | 6.48 ± 0.05 | 96 | |
| MRSS | 0.03 a | 3.71 ± 0.10 | 31.3 ± 0.53 | 5.77 ± 0.17 | 7.91 ± 0.68 | 48.7 | 50.9 ± 0.56 | 96 | |
| MRBS | 0.00 a | 0.99 ± 0.09 | 4.26 ± 0.03 | 1.20 a | 6.39 ± 0.05 | 13.4 | 12.7 ± 0.31 | 106 | |
| Zn | TU 171 | 0.05 a | 0.60 ± 0.01 | 1.14 ± 0.07 | 3.15 ± 0.32 | 17.0 ± 0.77 | 21.9 | 20.8 ± 0.81 | 105 |
| TU 169 | 0.04 a | 0.36 ± 0.03 | 1.59 ± 0.14 | 3.93 ± 0.3 | 18.5 ± 0.5 | 24.4 | 24.9 ± 0.40 | 98 | |
| MRSS | 1.80 ± 0.01 | 5.05 ± 0.18 | 33.4 ± 0.73 | 6.97 ± 0.21 | 52.8 ± 1.16 | 100.0 | 105 ± 2.30 | 95 | |
| MRBS | b | 1.28 ± 0.03 | 8.07 ± 0.08 | 2.03 ± 0.08 | 23.7 ± 0.17 | 35.3 | 36.4 ± 0.34 | 97 | |
| Co | TU 171 | 0.03 a | 0.15 a | 0.35 ± 0.02 | 0.89 ± 0.02 | 2.35 ± 0.05 | 3.74 | 3.79 ± 0.07 | 99 |
| TU 169 | 0.03 a | 0.14 ± 0.02 | 0.52 ± 0.03 | 1.05 ± 0.10 | 2.82 ± 0.11 | 4.55 | 4.69 ± 0.05 | 97 | |
| MRSS | 0.02 a | 0.28 a | 6.11 ± 0.23 | 0.92 ± 0.03 | 5.04 ± 0.24 | 12.4 | 11.9 ± 0.27 | 104 | |
| MRBS | 0.01 a | 0.03 a | 3.78 ± 0.08 | 1.05 ± 0.02 | 4.03 ± 0.11 | 8.90 | 8.88 ± 0.20 | 100 | |
| Ni | TU 171 | 0.02 a | 0.22 a | 0.86 ± 0.05 | 2.22 ± 0.08 | 5.93 ± 0.21 | 9.25 | 8.97 ± 0.54 | 103 |
| TU 169 | 0.02 a | 0.22 ± 0.01 | 1.01 ± 0.05 | 2.41 ± 0.18 | 6.79 ± 0.12 | 10.5 | 10.1 ± 0.29 | 104 | |
| MRSS | 0.16 a | 0.44 ± 0.02 | 5.84 ± 0.14 | 2.46 ± 0.08 | 24.2 ± 0.34 | 33.1 | 32.3 ± 0.84 | 103 | |
| MRBS | b | 0.15 a | 4.34 ± 0.10 | 2.00 ± 0.05 | 12.9 ± 0.44 | 19.4 | 19.4 ± 0.19 | 100 | |
| Cu | TU 171 | 0.03 a | 0.14 a | 0.16 a | 0.87 ± 0.04 | 2.88 ± 0.13 | 4.07 | 4.01 ± 0.28 | 102 |
| TU 169 | 0.02 a | 0.12 ± 0.02 | 0.21 ± 0.02 | 0.80 ± 0.03 | 2.81 ± 0.18 | 3.95 | 4.02 ± 0.33 | 98 | |
| MRSS | 0.55 ± 0.01 | 5.45 ± 0.01 | 24.3 ± 0.34 | 13.2 ± 0.13 | 33.9 ± 1.53 | 77.4 | 79.0 ± 1.03 | 98 | |
| MRBS | 0.06 a | 0.32 ± 0.01 | 1.20 ± 0.02 | 3.05 ± 0.07 | 27.8 ± 0.61 | 32.5 | 31.7 ± 0.76 | 103 |
| Fraction | Oldest Pre-Industrial Deposits | Youngest Pre-Industrial Deposits | MRSS | MRBS |
|---|---|---|---|---|
| 1 | Co > Cu > Ni > Zn > Pb | Co > Cu > Ni > Zn | Zn > Cu > Ni > Co > Pb | Cu > Co > Pb |
| (0.69) (0.59) (0.24) (0.2) (0.12) | (0.57) (0.43) (0.20) (0.15) | (1.7) (0.70) (0.48) (0.13) (0.06) | (0.18) (0.03) (0.02) | |
| 2 | Co > Cu > Zn > Ni > Pb | Co > Cu > Pb > Ni > Zn | Cu > Pb > Zn > Co > Ni | Pb > Co > Zn > Ni > Cu |
| (3.9) (3.3) (2.7) (2.4) (2.23) | (3.3) (2.9) (2.7) (2.2) (1.5) | (7.6) (6.9) (5.0) (2.25) (1.33) | (7.7) (3.6) (0.99) (0.76) (0.33) | |
| 3 | Ni > Co > Pb > Zn > Cu | Co > Ni > Pb > Zn > Cu | Pb > Co > Zn > Cu > Ni | Co > Pb > Zn > Ni > Cu |
| (9.3) (9.1) (8.8) (5.2) (3.7) | (11.3) (9.7) (6.7) (6.5) (5.1) | (64) (49) (33) (31) (18) | (42.0) (33.0) (22.8) (22.4) (3.69) | |
| 4 | Ni > Co > Cu > Zn > Pb | Ni > Co > Cu > Zn > Pb | Cu > Pb > Ni > Co > Zn | Co > Ni > Cu > Pb > Zn |
| (24) (23.7) (20.1) (14.4) (14) | (23.1) (23) (19.7) (16.1) (8.2) | (16.8) (11.9) (7.4) (7.3) (7) | (11.8) (10.3) (9.4) (9.3) (5.7) |
| SS (1973) a | SS (1974) b | SS (1982) c | SS (1991) d | SS-BCLA e | Colloids-BCLA f | SS-North America Rivers g | SS-Ave. World Rivers g | Delta Sediments h | Pre-Industrial Metals i | Upper Crust g | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pb | 45.5 | 46 | 32 ± 3 | 27–34 | 30.3 ± 3.1 | 38, 49 | 22 | 61.1 | 35.1 | 15.1 | 132 |
| Zn | 184 | 193 | 160 ± 27.5 | 183, 212 | 137 | 208 | 160 | 134 | |||
| Co | 21.2 | 21 | 15 | 22.5 | 18.9 | 11.4 | 20 | ||||
| Ni | 55.6 | 55 | 50 | 74.5 | 39.3 | 20 | |||||
| Cu | 42.3 | 45 | 50.7 ± 5.9 | 62, 72 | 34 | 75.9 | 29.2 | 24.2 | 25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akintomide, O.A.; Adebayo, S.A.; Trahan, A.S.; Chamberlain, E.; Johannesson, K.H. Investigating the Potential Impact of Louisiana Coastal Restoration on the Trace Metal Geochemistry of Constructed Marshlands. Soil Syst. 2020, 4, 55. https://doi.org/10.3390/soilsystems4030055
Akintomide OA, Adebayo SA, Trahan AS, Chamberlain E, Johannesson KH. Investigating the Potential Impact of Louisiana Coastal Restoration on the Trace Metal Geochemistry of Constructed Marshlands. Soil Systems. 2020; 4(3):55. https://doi.org/10.3390/soilsystems4030055
Chicago/Turabian StyleAkintomide, Omolola A., Segun A. Adebayo, Alexandra S. Trahan, Elizabeth Chamberlain, and Karen H. Johannesson. 2020. "Investigating the Potential Impact of Louisiana Coastal Restoration on the Trace Metal Geochemistry of Constructed Marshlands" Soil Systems 4, no. 3: 55. https://doi.org/10.3390/soilsystems4030055
APA StyleAkintomide, O. A., Adebayo, S. A., Trahan, A. S., Chamberlain, E., & Johannesson, K. H. (2020). Investigating the Potential Impact of Louisiana Coastal Restoration on the Trace Metal Geochemistry of Constructed Marshlands. Soil Systems, 4(3), 55. https://doi.org/10.3390/soilsystems4030055