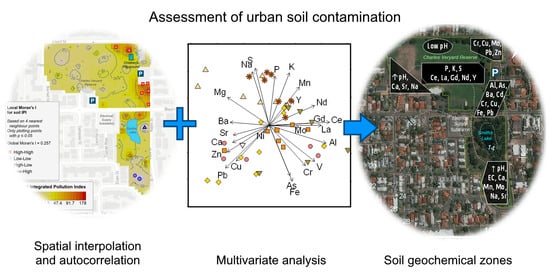
Spatial Analysis of Soil Trace Element Contaminants in Urban Public Open Space, Perth, Western Australia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling
2.3. Chemical Analysis
2.4. Statistical and Numerical Analysis
3. Results
3.1. Bulk Chemical Analyses of Surface Soil
3.2. Spatial Distributions in Surface Soil
3.3. Relationships between Soil Elements
3.4. Depth Distributions of As, Cr, Cu, Ni, Pb, and Zn
4. Discussion
4.1. Concentrations of Potential Contaminants
4.2. Spatial Patterns of Potential Contaminants in Surface Soil
4.3. Associations of Potential Contaminants
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demiguel, E.; Degrado, M.J.; Llamas, J.F.; Martindorado, A.; Mazadiego, L.F. The overlooked contribution of compost application to the trace element load in the urban soil of Madrid (Spain). Sci. Total Environ. 1998, 215, 113–122. [Google Scholar] [CrossRef]
- Chen, T.B.; Wong, J.W.C.; Zhou, H.Y.; Wong, M.H. Assessment of trace metal distribution and contamination in surface soils of Hong Kong. Environ. Pollut. 1997, 96, 61–68. [Google Scholar] [CrossRef]
- Gaw, S.K.; Wilkins, A.L.; Kim, N.D.; Palmer, G.T.; Robinson, P. Trace element and ΣDDT concentrations in horticultural soils from the Tasman, Waikato and Auckland regions of New Zealand. Sci. Total Environ. 2006, 355, 31–47. [Google Scholar] [CrossRef]
- Tarzia, M.; De Vivo, B.; Somma, R.; Ayuso, R.A.; McGill, R.A.R.; Parrish, R.R. Anthropogenic vs. natural pollution: An environmental study of an industrial site under remediation (Naples, Italy). Geochem. Explor. Environ. Anal. 2002, 2, 45–56. [Google Scholar] [CrossRef]
- Appleyard, S.; Wong, S.; Willis-Jones, B.; Angeloni, J.; Watkins, R. Groundwater acidification caused by urban development in Perth, Western Australia: Source, distribution, and implications for management. Aust. J. Soil Res. 2004, 42, 579–585. [Google Scholar] [CrossRef]
- Orndorff, Z.W.; Daniels, W.L.; Fanning, D.S. Reclamation of acid sulfate soils using lime-stabilized biosolids. J. Environ. Qual. 2008, 37, 1447–1455. [Google Scholar] [CrossRef]
- Grundy, S.L.; Bright, D.A.; Dushenko, W.T.; Dodd, M.; Englander, S.; Johnston, K.; Pier, D.; Reimer, K.J. Dioxin and furan signatures in northern Canadian soils: Correlation to source signatures using multivariate unmixing techniques. Chemosphere 1997, 34, 1203–1219. [Google Scholar] [CrossRef]
- Riemann, U. Impacts of urban growth on surface water and groundwater quality in the City of Dessau, Germany. In Impacts of Urban Growth on Surface Water and Groundwater Quality; Ellis, B., Ed.; IAHS-AISH Publication No. 259; International Association of Hydrological Sciences Press: Wallingford, UK, 1999; pp. 307–314. [Google Scholar]
- Liu, E.; Yan, T.; Birch, G.; Zhu, Y. Pollution and health risk of potentially toxic metals in urban road dust in Nanjing, a mega-city of China. Sci. Total Environ. 2014, 476–477, 522–531. [Google Scholar] [CrossRef]
- Henderson, F.M.; Xia, Z.G. SAR applications in human settlement detection, population estimation and urban land use pattern analysis: A status report. IEEE Trans. Geosci. Remote Sens. 1997, 35, 79–85. [Google Scholar] [CrossRef]
- Huo, X.N.; Zhang, W.W.; Sun, D.F.; Li, H.; Zhou, L.D.; Li, B.G. Spatial pattern analysis of heavy metals in Beijing agricultural soils based on spatial autocorrelation statistics. Int. J. Environ. Res. Public Health 2011, 8, 2074–2089. [Google Scholar] [CrossRef] [Green Version]
- Anselin, L. Local Indicators of Spatial Association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Hojati, S. Use of spatial statistics to identify hotspots of lead and copper in selected soils from north of Khuzestan Province, southwestern Iran. Arch. Agron. Soil Sci. 2019, 65, 654–669. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, L.; Xu, W.; Ledwith, V. Use of local Moran’s I and GIS to identify pollution hotspots of Pb in urban soils of Galway, Ireland. Sci. Total Environ. 2008, 398, 212–221. [Google Scholar] [CrossRef] [PubMed]
- City of Vincent. Thematic History; City of Vincent: Leederville, WA, Australia, 2008.
- Claise Brook Catchment Group. Restoration of Smith’s Lake. Available online: http://www.cbcg.org.au/projects_smiths.html (accessed on 31 October 2017).
- Western Australian Land Information Authority. Landgate Map Viewer Plus. Available online: https://maps.landgate.wa.gov.au/maps-landgate/registered/. https://www0.landgate.wa.gov.au/maps-and-imagery/imagery/aerial-photography/aerial (accessed on 29 June 2021).
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods—Australasia; CSIRO Publishing: Clayton, VIC, Australia, 2010. [Google Scholar]
- U.S. EPA. Method 3050B: Acid digestion of sediments, sludges, and soils test. In Methods for Evaluating Solid Waste, Physical/Chemical Methods; EPA Publication SW-846; U.S. EPA: Washington, DC, USA, 2007. [Google Scholar]
- Lynch, J. Additional provisional elemental values for LKSD-1, LKSD-2, LKSD-3, LKSD-4, STSD-1, STSD-2, STSD-3 and STSD-4. Geostand. Newsl. 1999, 23, 251–260. [Google Scholar] [CrossRef]
- Long, X.X.; Yang, X.E.; Ni, W.Z.; Ye, Z.Q.; He, Z.L.; Calvert, D.V.; Stoffella, J.P. Assessing zinc thresholds for phytotoxicity and potential dietary toxicity in selected vegetable crops. Commun. Soil Sci. Plant Anal. 2003, 34, 1421–1434. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing (Version 4.0.3); R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 11 August 2021).
- Pohlert, T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended (R Package). 2018. Available online: https://cran.r-project.org/web/packages/PMCMRplus/index.html (accessed on 11 August 2021).
- Reimann, C.; Filzmoser, P.; Garrett, R.G.; Dutter, R. Statistical Data Analysis Explained: Applied Environmental Statistics with R, 1st ed.; John Wiley & Sons: Chichester, UK, 2008; p. 343. [Google Scholar]
- Fellows, I. OpenStreetMap: Access to Open street Map Raster Images, Using the JMapViewer Library by Jan Peter Stotz. 0.3.3; (R Package Version 0.3.4). 2019. Available online: http://CRAN.R-project.org/package=OpenStreetMap (accessed on 11 August 2021).
- Google. Getting Started | Google Maps Elevation API | Google Developers. Available online: https://developers.google.com/maps/documentation/elevation (accessed on 9 October 2017).
- Cooley, D. Googleway: Accesses Google Maps APIs to Retrieve Data and Plot Maps; R Package Version 2.0.0; 2017. Available online: https://cran.r-project.org/web/packages/googleway/index.html (accessed on 11 August 2021).
- Akima, H.; Gebhardt, A.; Petzoldt, T.; Maechler, M. akima: Interpolation of Irregularly Spaced Data. R Package Version 0.5–11. 2013. Available online: http://CRAN.R-project.org/package=akima (accessed on 11 August 2021).
- Kalogirou, S. lctools: Local Correlation, Spatial Inequalities, Geographically Weighted Regression and Other Tools. R Package Version 0.2–8. Available online: https://CRAN.R-project.org/package=lctools (accessed on 28 May 2021).
- Pebesma, E.; Bivand, R. sp: Classes and Methods for Spatial Data; R Package Version 1.4-4; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Pebesma, E.J.; Graeler, B. gstat: Spatial and Spatio-Temporal Geostatistical Modelling, Prediction and Simulation; R Package Version 2.0-7; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Webster, R.; Oliver, M.A. How large a sample is needed to estimate the regional variogram adequately? Geostat. Troia’92 1993, 1, 155–166. [Google Scholar]
- Sun, Y.; Zhou, Q.; Xie, X.; Liu, R. Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang, China. J. Hazard. Mater. 2010, 174, 455–462. [Google Scholar] [CrossRef]
- DWER. Final Report: Review of the Uncontaminated Fill Thresholds in Table 6 of the Landfill Waste Classification and Waste Definitions 1996 (as Amended 2018); Department of Water and Environmental Regulation, Government of Western Australia: Joondalup, WA, Australia, 2019.
- Rate, A.W. Multielement geochemistry identifies the spatial pattern of soil and sediment contamination in an urban parkland, Western Australia. Sci. Total Environ. 2018, 627, 1106–1120. [Google Scholar] [CrossRef]
- National Environment Protection Council. Schedule B (1): Guideline on the investigation levels for soil and groundwater. In National Environment Protection (Assessment of Site Contamination) Measure (Amended); Commonwealth of Australia: Canberra, Australia, 2013. [Google Scholar]
- National Environment Protection Council. Schedule B (1): Guideline on the investigation levels for soil and groundwater. In National Environment Protection (Assessment of Site Contamination) Measure; Commonwealth of Australia: Canberra, Australia, 1999. [Google Scholar]
- Rimmer, D.L.; Younger, A. Land reclamation after coal-mining operations. In Contaminated Land and Its Reclamation; Hester, R.E., Harrison, R.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 1997; pp. 73–90. [Google Scholar]
- Callender, E.; Rice, K.C. The urban environmental gradient: Anthropogenic influences on the spatial and temporal distributions of lead and zinc in sediments. Environ. Sci. Technol. 2000, 34, 232–238. [Google Scholar] [CrossRef]
- Charlesworth, S.; de Miguel, E.; Ordóñez, A. A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environ. Geochem. Health 2011, 33, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Smolders, E.; Oorts, K.; van Sprang, P.; Schoeters, I.; Janssen, C.R.; McGrath, S.P.; McLaughlin, M.J. Toxicity of trace metals in soil as affected by soil type and aging after contamination: Using calibrated bioavailability models to set ecological soil standards. Environ. Toxicol. Chem. 2009, 28, 1633–1642. [Google Scholar] [CrossRef]
- Pietrzak, U.; McPhail, D.C. Copper accumulation, distribution and fractionation in vineyard soils of Victoria, Australia. Geoderma 2004, 122, 151–166. [Google Scholar] [CrossRef]
- Harrison, R.M.; Laxen, D.P.H.; Wilson, S.J. Chemical associations of lead, cadmium, copper, and zinc in street dusts and roadside soils. Environ. Sci. Technol. 1981, 15, 1378–1383. [Google Scholar] [CrossRef]
- Hamon, R.E.; McLaughlin, M.J.; Gilkes, R.J.; Rate, A.W.; Zarcinas, B.; Robertson, A.; Cozens, G.; Radford, N.; Bettenay, L. Geochemical indices allow estimation of heavy metal background concentrations in soils. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef] [Green Version]
- Tanner, P.A.; Ma, H.L.; Yu, P.K.N. Fingerprinting metals in urban street dust of Beijing, Shanghai, and Hong Kong. Environ. Sci. Technol. 2008, 42, 7111–7117. [Google Scholar] [CrossRef]
- Davis, A.P.; Shokouhian, M.; Ni, S. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere 2001, 44, 997–1009. [Google Scholar] [CrossRef]
- Jim, C.Y. Urban soil characteristics and limitations for landscape planting in Hong Kong. Landsc. Urban Plan. 1998, 40, 235–249. [Google Scholar] [CrossRef]
- Mielke, H.W.; Laidlaw, M.A.S.; Gonzales, C. Lead (Pb) legacy from vehicle traffic in eight California urbanized areas: Continuing influence of lead dust on children’s health. Sci. Total Environ. 2010, 408, 3965–3975. [Google Scholar] [CrossRef]
- Béze, L.E.; Rose, J.; Mouillet, V.; Farcas, F.; Masion, A.; Chaurand, P.; Bottero, J.-Y. Location and evolution of the speciation of vanadium in bitumen and model of reclaimed bituminous mixes during ageing: Can vanadium serve as a tracer of the aged and fresh parts of the reclaimed asphalt pavement mixture? Fuel 2012, 102, 423–430. [Google Scholar] [CrossRef]
- Conacher, J. Historic Land Use Survey of the Claisebrook Catchment; The University of Western Australia for the Claisebrook Catchment Group: Crawley, Australia, 2000; p. 61.
- City of Vincent. Charles Veryard Reserve, Place Number 17957. In inHerit—Places Database; Heritage Council of WA: Perth, WA, Australia, 2007. [Google Scholar]
- Ljung, K.; Maley, F.; Cook, A. Canal estate development in an acid sulfate soil-Implications for human metal exposure. Landsc. Urban Plan. 2010, 97, 123–131. [Google Scholar] [CrossRef]











| Statistic | pH | EC | Major Element Concentration (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (µS/cm) | Al | Ca | Fe | K | Mg | Na | P | S | ||
| Surface soil (random-in-grid samples, 2017) | ||||||||||
| Mean | 6.92 | 183 | 2667 | 5083 | 2695 | 161 | 425 | 130 | 244 | 309 |
| Std. Dev. | 0.63 | 146 | 758 | 7082 | 939 | 75.3 | 285 | 69 | 123 | 186 |
| Minimum | 5.28 | 29.1 | 396 | 283 | 1205 | 41.1 | 57.3 | 27.8 | 18.5 | 43 |
| Median | 6.84 | 151 | 2637 | 1813 | 2530 | 150 | 347 | 118 | 221 | 280 |
| Maximum | 8.63 | 835 | 4722 | 30,472 | 5640 | 424 | 1471 | 410 | 596 | 1293 |
| No of valid analyses | 73 | 58 | 68 | 68 | 68 | 68 | 68 | 68 | 68 | 68 |
| Soil profiles to ≤ 100 cm (2018) | ||||||||||
| Mean | 7.67 | 116 | 3214 | 11,061 | 4430 | 173 | 548 | 148 | 146 | 518 |
| Std. Dev. | 1.96 | 61 | 2168 | 14,254 | 3358 | 100 | 505 | 100 | 137 | 334 |
| Minimum | 4.48 | 7.92 | 149 | 38 | 218 | 26 | 14 | 11 | 4 | 48 |
| Median | 7.98 | 83 | 2880 | 7894 | 3593 | 134 | 450 | 127 | 140 | 293 |
| Maximum | 9.40 | 860 | 10,353 | 57,265 | 22,180 | 655 | 2365 | 513 | 386 | 4929 |
| No of valid analyses | 84 | 83 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 83 |
| Concentrations (mg/kg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistic | As | Ba | Cd | Cr | Cu | Mn | Mo | Ni | Pb | Sr | V | Zn |
| Surface soil (random-in-grid samples, 2017) | ||||||||||||
| Mean | 2.58 | 15.7 | 0.11 | 7.33 | 8.69 | 39.5 | 0.23 | 2.58 | 25.7 | 27.8 | 5.38 | 55.2 |
| Std. Dev. | 1.09 | 7.38 | 0.1 | 2.29 | 11 | 23.7 | 0.1 | 2.83 | 31.7 | 40.3 | 1.66 | 56.5 |
| Minimum | 0.9 | 6.2 | 0.02 | 1.17 | 2.13 | 2.68 | 0.07 | 0.25 | 3.23 | 2.4 | 1.28 | 5.59 |
| Median | 2.3 | 14.5 | 0.08 | 7.32 | 5.25 | 34.5 | 0.22 | 2.13 | 14.7 | 10.3 | 5.35 | 34.7 |
| Maximum | 5.86 | 41.7 | 0.66 | 13.8 | 67.5 | 127 | 0.6 | 21.3 | 174 | 186 | 10.6 | 304 |
| No of analyses | 68 | 68 | 62 | 68 | 68 | 68 | 68 | 68 | 68 | 68 | 68 | 68 |
| No > HIL(C) 1 | 0 | - | 0 | 0 | 0 | 0 | - | 0 | 0 | - | - | 0 |
| No > EIL 3 | 0 | 0 | 0 | 0 | 0 | 0 | - | 0 | 0 | - | 0 | 3 |
| Soil profiles to ≤ 100 cm (2018) | ||||||||||||
| Mean | 3.28 | 25.2 | 0.18 | 9.09 | 41.4 | 28.0 | 0.48 | 5.39 | 72.7 | 62.3 | 8.38 | 117 |
| Std. Dev. | 2.79 | 26.2 | 0.31 | 6.96 | 66.6 | 21.7 | 0.81 | 20.7 | 98.8 | 85.1 | 5.56 | 191 |
| Minimum | 0.6 | 1.9 | 0.01 | 0.2 | 2.8 | 1.5 | 0.06 | 0.6 | 3.5 | 1.8 | 0.3 | 3.3 |
| Median | 2.5 | 15.3 | 0.06 | 8.1 | 15.1 | 24.3 | 0.21 | 1.97 | 32.2 | 34.3 | 7.2 | 43.2 |
| Maximum | 14.6 | 139 | 1.6 | 34.4 | 356 | 97.9 | 5.63 | 181 | 568 | 391 | 30.4 | 1155 |
| No of analyses | 83 | 85 | 79 | 85 | 67 | 85 | 78 | 75 | 84 | 80 | 85 | 84 |
| No > HIL(C) 1 | 0 | - | 0 | 0 | 0 | 0 | - | 0 | 0 | - | - | 0 |
| No > EIL 3 | 0 | 0 | 0 | 0 | 8 | 0 | - | 1 | 1 | 0 | 0 | 14 |
| Variable | Moran’s I | p-Value | Number of Points with Significant Local Moran’s I | Location (and Number of Points) of High-High LOCAL Moran’s I Clusters a |
|---|---|---|---|---|
| pH | 0.440 | >0.001 | 13 | CVR-SW (2), CVR-SE (3), SLR-S (4) |
| EC | 0.157 | 0.035 | 7 | SLR-S (3) |
| Al | 0.014 | 0.691 | 4 | CVR-SE (2) b |
| As | 0.228 | 0.002 | 6 | CVR-SE (4) |
| Ca | 0.074 | 0.254 | 6 | SLR-S (2) |
| Cr | 0.016 | 0.672 | 4 | CVR-SE (1) |
| Cu | 0.331 | >0.001 | 9 | CVR-NE (3), CVR-SE (5) |
| Fe | 0.142 | 0.044 | 11 | CVR-NE (1), CVR-SE (5) |
| Ni | 0.047 | 0.426 | 5 | CVR-SE (1) b |
| Pb | 0.324 | >0.001 | 11 | CVR-SE (5), SLR-N (2) |
| Zn | 0.209 | 0.004 | 10 | CVR-NE (2), CVR-SE (4) |
| IPI | 0.257 | >0.001 | 10 | CVR-NE (1), CVR-SE (5) |
| Variable | P (K-W) 1 | Mean in Each Zone 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CVR- Centre | CVR-NE | CVR-NW | CVR-SE | CVR-SW | SLR-N | SLR-S | Other | ||
| pH | 0.0001 | 6.58 a | 6.65 ab | 6.24 a | 7.34 bc | 7.35 bc | 6.88 abc | 7.38 c | 6.66 abc |
| EC | 0.013 | 155 ab | 90.7 ab | 273 a | 159 ab | 232 a | 68.1 b | 265 ab | 143 ab |
| Al | 0.028 | 2530 ab | 2857 ab | 2285 a | 3373 b | 2621 ab | 1960 ab | 3011 ab | 2489 ab |
| As | 0.004 | 1.91 a | 2.46 ab | 1.96 a | 3.79 b | 2.17 a | 2.99 ab | 3.04 ab | 2.38 ab |
| Ca | 0.004 | 1766 abc | 3511 abc | 4534 abc | 6710 abc | 7472 ab | 3570 ac | 11,450 b | 1343 c |
| Cr | 0.020 | 6.98 ab | 8.47 ab | 6.62 a | 9.42 b | 7.18 ab | 5.85 ab | 7.51 ab | 6.20 a |
| Cu | 0.010 | 5.10 ab | 16.7 ab | 3.57 a | 17.6 b | 5.36 ab | 8.43 ab | 8.34 ab | 4.52 ab |
| Fe | 0.041 | 2377 ab | 2936 ab | 2071 a | 3623 b | 2418 ab | 2680 ab | 2861 ab | 2535 ab |
| Ni | 0.300 | ns | ns | ns | ns | ns | ns | ns | ns |
| Pb | 0.043 | 11.7 a | 31.2 ab | 15.4 ab | 56.9 b | 15.3 ab | 47.8 ab | 15.7 a | 16.5 ab |
| Zn | 0.255 | ns | ns | ns | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rate, A.W. Spatial Analysis of Soil Trace Element Contaminants in Urban Public Open Space, Perth, Western Australia. Soil Syst. 2021, 5, 46. https://doi.org/10.3390/soilsystems5030046
Rate AW. Spatial Analysis of Soil Trace Element Contaminants in Urban Public Open Space, Perth, Western Australia. Soil Systems. 2021; 5(3):46. https://doi.org/10.3390/soilsystems5030046
Chicago/Turabian StyleRate, Andrew W. 2021. "Spatial Analysis of Soil Trace Element Contaminants in Urban Public Open Space, Perth, Western Australia" Soil Systems 5, no. 3: 46. https://doi.org/10.3390/soilsystems5030046
APA StyleRate, A. W. (2021). Spatial Analysis of Soil Trace Element Contaminants in Urban Public Open Space, Perth, Western Australia. Soil Systems, 5(3), 46. https://doi.org/10.3390/soilsystems5030046