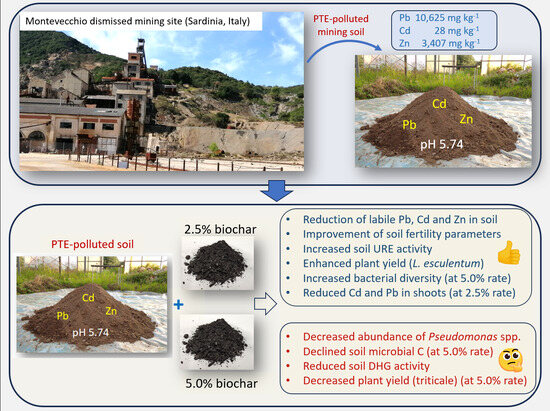
Sustainable Restoration of Soil Functionality in PTE-Affected Environments: Biochar Impact on Soil Chemistry, Microbiology, Biochemistry, and Plant Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Origin, Biochar, and Mesocosms Set Up
2.2. Soil Chemical Analyses
2.3. Mobility of Pb, Cd, and Zn in Soil
2.4. Culturable Microorganisms and Soil Microbial Biomass
2.5. Molecular Analysis of the Soil Bacterial Community through 16S rRNA Gene Amplicon Sequencing
2.5.1. Bioinformatics
2.5.2. Biostatistics
2.6. Soil Enzyme Activities and Community Level Physiological Profile
2.7. Plant Growth and PTE Uptake
2.8. Data Analysis
3. Results and Discussion
3.1. Influence of Biochar on the Chemical Characteristics of the Polluted Soil
3.2. Influence of Biochar on the Mobility of Pb, Cd and Zn in Soil
3.3. Influence of Biochar on Culturable Microorganisms and Soil Microbial Biomass
3.4. Influence of Biochar on the Structure of Soil Bacterial Community
3.5. Influence of Biochar on Soil Enzyme Activities and Community Level Physiological Profile
3.6. Influence of Biochar on Plant Growth and PTE Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef] [PubMed]
- Diquattro, S.; Garau, G.; Mangia, N.P.; Drigo, B.; Lombi, E.; Vasileiadis, S.; Castaldi, P. Mobility and potential bioavailability of antimony in contaminated soils: Short-term impact on microbial community and soil biochemical functioning. Ecotoxicol. Environ. Saf. 2020, 196, 110576. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Lu, X.; Wang, L.; Liang, T.; Fan, X.; Yang, Y.; Lei, K.; Zuo, L.; Fan, P.; Bolan, N.; et al. Potentially toxic elements in surface fine dust of residence communities in valley industrial cities. Environ. Pollut. 2023, 327, 121523. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Porceddu, A.; Sanna, M.; Silvetti, M.; Castaldi, P. Municipal solid wastes as a resource for environmental recovery: Impact of water treatment residuals and compost on the microbial and biochemical features of As and trace metal-polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Roggero, P.P.; Diquattro, S.; Garau, M.; Pinna, M.V.; Castaldi, P. Innovative amendments derived from industrial and municipal wastes enhance plant growth and soil functions in potentially toxic elements-polluted environments. Ital. J. Agron. 2021, 16, 1777. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A.; Chakraborty, S.; Vithanage, M.; Biswas, J.K. Multifaceted applications of biochar in environmental management: A bibliometric profile. Biochar 2023, 5, 11. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Pinna, M.V.; Lauro, G.P.; Diquattro, S.; Garau, M.; Senette, C.; Castaldi, P.; Garau, G. Softwood-derived biochar as a green material for the recovery of environmental media contaminated with potentially toxic elements. Water Air Soil Pollut. 2022, 233, 152. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Konvalina, P.; Moudrý, J.; Bárta, J.; Kopecký, M.; Teodorescu, R.I.; Bucur, R.D. Comparative Influence of Biochar and Zeolite on Soil Hydrological Indices and Growth Characteristics of Corn (Zea mays L.). Water 2022, 14, 3506. [Google Scholar] [CrossRef]
- Lomaglio, T.; Hattab-Hambli, N.; Bret, A.; Miard, F.; Trupiano, D.; Scippa, G.S.; Motelica-Heino, M.; Bourgerie, S.; Morabito, D. Effect of biochar amendments on the mobility and (bio) availability of As, Sb and Pb in a contaminated mine technosol. J. Geochem. Explor. 2017, 182, 138–148. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Mosa, A.; Natasha; Jeyasundar, P.G.S.A.; Hassan, N.E.E.; Yang, X.; Antoniadis, V.; Li, R.; Wang, J.; Zhang, T.; et al. Pros and Cons of Biochar to Soil Potentially Toxic Element Mobilization and Phytoavailability: Environmental Implications. Earth Syst. Environ. 2023, 7, 321–345. [Google Scholar] [CrossRef]
- Sachdeva, S.; Kumar, R.; Sahoo, P.K.; Nadda, A.K. Recent advances in biochar amendments for immobilization of heavy metals in an agricultural ecosystem: A systematic review. Environ. Pollut. 2023, 319, 120937. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, B.; Peng, Y.; Gao, X.; Fan, B.; Chen, Q. A meta-analysis of heavy metal bioavailability response to biochar aging: Importance of soil and biochar properties. Sci. Total Environ. 2021, 756, 144058. [Google Scholar] [CrossRef] [PubMed]
- Zama, E.F.; Reid, B.J.; Arp, H.P.H.; Sun, G.X.; Yuan, H.Y.; Zhu, Y.G. Advances in research on the use of biochar in soil for remediation: A review. J. Soils Sediments 2018, 18, 2433–2450. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Beesley, L.; Dickinson, N. Carbon and trace element fluxes in the pore water of an urban soil following greenwaste compost, woody and biochar amendments, inoculated with the earthworm Lumbricus terrestris. Soil Biol. Biochem. 2011, 43, 188–196. [Google Scholar] [CrossRef]
- Abou Jaoude, L.; Castaldi, P.; Nassif, N.; Pinna, M.V.; Garau, G. Biochar and compost as gentle remediation options for the recovery of trace elements-contaminated soils. Sci. Total Environ. 2020, 711, 134511. [Google Scholar] [CrossRef]
- Garau, M.; Garau, G.; Sizmur, T.; Coole, S.; Castaldi, P.; Pinna, M.V. Biochar and Eisenia fetida (Savigny) promote sorghum growth and the immobilization of potentially toxic elements in contaminated soils. Appl. Soil Ecol. 2023, 182, 104697. [Google Scholar] [CrossRef]
- Manzano, R.; Diquattro, S.; Roggero, P.P.; Pinna, M.V.; Garau, G.; Castaldi, P. Addition of softwood biochar to contaminated soils decreases the mobility, leachability and bioaccesibility of potentially toxic elements. Sci. Total Environ. 2020, 739, 139946. [Google Scholar] [CrossRef]
- El-Naggar, A.; Shaheen, S.M.; Ok, Y.S.; Rinklebe, J. Biochar affects the dissolved and colloidal concentrations of Cd, Cu, Ni, and Zn and their phytoavailability and potential mobility in a mining soil under dynamic redox-conditions. Sci. Total Environ. 2018, 624, 1059–1071. [Google Scholar] [CrossRef]
- Lahlali, R.; Ibrahim, D.S.; Belabess, Z.; Roni, Z.K.; Radouane, N.; Vicente, C.S.; Menéndez, E.; Mokrini, F.; Barka, E.A.; Mota, M.G.D.M.E.; et al. High-throughput molecular technologies for unraveling the mystery of soil microbial community: Challenges and future prospects. Heliyon 2021, 7, e08142. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.M.; Purchase, D.; Chen, X.W. Microbial communities and functions contribute to plant performance under various stresses. Front. Microbiol. 2022, 13, 992909. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Yao, G.; Lin, Z.; Xu, L.; Jiang, Y.; Jin, Z.; Shan, S.; Ping, L. Advances in the effects of biochar on microbial ecological function in soil and crop quality. Sustainability 2022, 14, 10411. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef] [PubMed]
- Anders, E.; Watzinger, A.; Rempt, F.; Kitzler, B.; Wimmer, B.; Zehetner, F.; Stahr, K.; Zechmeister-Boltenstern, S.; Soja, G. Biochar affects the structure rather than the total biomass of microbial communities in temperate soils. Agric. Food Sci. 2013, 22, 404–423. [Google Scholar] [CrossRef]
- Andrés, P.; Rosell-Melé, A.; Colomer-Ventura, F.; Denef, K.; Cotrufo, M.F.; Riba, M.; Alcañiz, J.M. Belowground biota responses to maize biochar addition to the soil of a Mediterranean vineyard. Sci. Total Environ. 2019, 660, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, D.; Liang, G.; Zhang, Q.; Ai, C.; Zhou, W. Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl. Soil Ecol. 2015, 96, 265–272. [Google Scholar] [CrossRef]
- Domene, X.; Hanley, K.; Enders, A.; Lehmann, J. Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Appl. Soil Ecol. 2015, 89, 10–17. [Google Scholar] [CrossRef]
- Dempster, D.N.; Gleeson, D.B.; Solaiman, Z.M.; Jones, D.L.; Murphy, D.V. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012, 354, 311–324. [Google Scholar] [CrossRef]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- El-Naggar, A.; El-Naggar, A.H.; Shaheen, S.M.; Sarkar, B.; Chang, S.X.; Tsang, D.C.; Rinklebe, J.; Ok, Y.S. Biochar composition-dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: A review. J. Environ. Manag. 2019, 241, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Laird, D.A.; Ahmedna, M.A.; Niandou, M.A.S. Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma 2010, 154, 281–288. [Google Scholar] [CrossRef]
- Garau, M.; Castaldi, P.; Patteri, G.; Roggero, P.P.; Garau, G. Evaluation of Cynara cardunculus L. and municipal solid waste compost for aided phytoremediation of multi potentially toxic element–contaminated soils. Environ. Sci. Pollut. Res. 2021, 28, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Nandillon, R.; Miard, F.; Bourgerie, S.; Morabito, D. Biochar assisted phytoremediation for metal(loid) contaminated soils. In Assisted Phytoremediation; Pandey, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–130. [Google Scholar] [CrossRef]
- Vacca, A.; Bianco, M.R.; Murolo, M.; Violante, P. Heavy metals in contaminated soils of the rio sitzerri floodplain (Sardinia, Italy): Characterization and impact on pedodiversity. Land Degrad. Develop. 2012, 23, 350–364. [Google Scholar] [CrossRef]
- Manca, P.P.; Massacci, G.; Mercante, C. Environmental management and metal recovery: Re-processing of mining waste at Montevecchio site (SW Sardinia). In 18th International Symposium on Environmental Issues and Waste Management in Energy and Mineral Production; Widzyk-Capehart, E., Hekmat, A., Singhal, R., Eds.; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Tan, K.H. Soil Sampling, Preparation, and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 169–170. [Google Scholar]
- Lomaglio, T.; Hattab-Hambli, N.; Miard, F.; Lebrun, M.; Nandillon, R.; Trupiano, D.; Scippa, G.S.; Gauthier, A.; Motelica-Heino, M.; Bourgerie, S.; et al. Cd, Pb, and Zn mobility and (bio)availability in contaminated soils from a former smelting site amended with biochar. Environ. Sci. Pollut. Res. 2018, 25, 25744–25756. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale Della Repubblica Italiana n. 84 del 10 aprile 2002. Metodi Ufficiali di Analisi Chimica dei Suoli. DM 11 Maggio 1992, suppl. G.U. 121, 25 maggio 1992. [Official Gazette of the Italian Republic No 84 of 10 April 2002, 2002. Official Methods of Chemical Analysis of Soils. DM 11 Maggio 1992, Suppl. G.U. 121, 25 May 1992]. Available online: https://www.gazzettaufficiale.it/eli/id/1992/05/25/092A2322/sg (accessed on 29 August 2023).
- Basta, N.; Gradwohl, R. Estimation of Cd, Pb and Zn bioavailability in smelter-contaminated soils by a sequential extraction procedure. J. Soil Contam. 2020, 9, 149–164. [Google Scholar] [CrossRef]
- Nunan, N.; Morgan, M.A.; Herlihy, M. Ultraviolet absorbance (280 nm) of compounds released from soil during chloroform fumigation as an estimate of the microbial biomass. Soil Biol. Biochem. 1998, 30, 1599–1603. [Google Scholar] [CrossRef]
- ISO 14240-2 Soil Quality—Determination of Soil Microbial Biomass—Part 2: Fumigation-Extraction Method. 1997. Available online: https://www.iso.org/obp/ui/#iso:std:iso:14240:-2:ed-1:v1:en (accessed on 20 August 2023).
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 2016, 1, e00009-15. [Google Scholar] [CrossRef]
- Comeau, A.M.; Douglas, G.M.; Langille, M.G.I. Microbiome helper: A custom and streamlined workflow for microbiome research. mSystems 2017, 2, e00127-16. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holme, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, Reference Index Version 4.3.1. R Foundation. 2023. Available online: https://www.r-project.org/ (accessed on 20 August 2023).
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Marcon, E.; Hérault, B. Entropart: An R package to measure and partition diversity. J. Stat. Softw. 2015, 67, 1–26. Available online: http://EconPapers.repec.org/RePEc:jss:jstsof:v:067:i08 (accessed on 20 August 2023). [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Gomez, E.; Ferreras, L.; Toresani, S. Soil bacterial functional diversity as influenced by organic amendment application. Bioresour. Technol. 2006, 97, 1484–1489. [Google Scholar] [CrossRef]
- Garau, G.; Castaldi, P.; Santona, L.; Deiana, P.; Melis, P. Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil. Geoderma 2007, 142, 47–57. [Google Scholar] [CrossRef]
- Decreto Legislativo 152/2006 (2006) Decreto Legislativo 3 Aprile 2006, n. 152 Norme in Materia Ambientale. Gazzetta Ufficiale Serie Generale n.88 del 14-04-2006—Supplemento Ordinario n. 96. [Legislative Decree No. 152/2006 (2006) Legislative Decree No. 152 of 3 April 2006 Environmental Regulations. Official Gazette General Series No. 88 of 14-04-2006—Ordinary Supplement No. 96]. Available online: https://www.gazzettaufficiale.it/dettaglio/codici/materiaAmbientale (accessed on 20 August 2023).
- Decreto Ministeriale 1 Marzo 2019 n. 46—Regolamento Relativo Agli Interventi di Bonifica, di Ripristino Ambientale e di Messa in Sicurezza, D’emergenza, Operativa e Permanente, Delle Aree Destinate Alla Produzione Agricola e All’allevamento, ai Sensi Dell’articolo 241 del Decreto Legislativo 3 Aprile 2006, n. 152 [Ministerial Decree (D.M.) 1 March 2019 n. 46—Regulation on the Remediation, Environmental Restoration and Safety Interventions, Emergency, Operational and Permanent, of Areas Intended for Agricultural Production and Livestock Farming, Pursuant to Article 241 of Legislative Decree No. 152 of 3 April 2006]. Available online: https://www.gazzettaufficiale.it/eli/id/2019/06/07/19G00052/sg (accessed on 20 August 2023).
- Gao, Y.; Fang, Z.; Van Zwieten, L.; Bolan, N.; Dong, D.; Quin, B.F.; Meng, J.; Li, F.; Wu, F.; Wang, H.; et al. A critical review of biochar-based nitrogen fertilizers and their effects on crop production and the environment. Biochar 2022, 4, 36. [Google Scholar] [CrossRef]
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.L.; Suravajhala, P.; Kishor, P.B.K. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. Biometals 2016, 29, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Sarmah, A.K.; Bordoloi, S.; Bolan, S.; Padhye, L.P.; Van Zwieten, L.; Sooriyakumar, P.; Khan, B.A.; Ahmad, M.; Solaiman, Z.M.; et al. Soil acidification and the liming potential of biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- De Lima Veloso, V.; da Silva, F.B.V.; dos Santos, N.M.; do Nascimento, C.W.A. Phytoattenuation of Cd, Pb, and Zn in a slag-contaminated soil amended with rice straw biochar and grown with energy maize. Environ. Manag. 2022, 69, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Olaniran, A.F.; Adenusi, T.T.; Aremu, C.; Ejue, W.S.; Iranloye, Y.M.; Gbadamosi, A.; Olayanju, A. Effects of cow dung and wood biochars and green manure on soil fertility and tiger nut (Cyperus esculentus L.) performance on a savanna Alfisol. Sci. Rep. 2020, 10, 21021. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-L.; Han, G.-M.; Lan, Y.; Liu, S.-N.; Gao, J.-P.; Yang, X.; Meng, J.; Chen, W.-F. Corn cob biochar increases soil culturable bacterial abundance without enhancing their capacities in utilizing carbon sources in Biolog Eco-plates. J. Integr. Agric. 2017, 16, 713–724. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Saleem, M.; Ali, M.Y.; Xiang, J. Biochar and earthworms synergistically improve soil structure, microbial abundance, activities and pyraclostrobin degradation. Appl. Soil Ecol. 2021, 168, 104154. [Google Scholar] [CrossRef]
- Neff, J.; Asner, G. Dissolved organic carbon in terrestrial ecosystems: Synthesis and a model. Ecosyst. 2001, 4, 29–48. [Google Scholar] [CrossRef]
- Azeem, M.; Jeyasundar, P.G.S.A.; Ali, A.; Riaz, L.; Khan, K.S.; Hussain, Q.; Kareem, H.A.; Abbas, F.; Latif, A.; Majrashi, A.; et al. Cow bone-derived biochar enhances microbial biomass and alters bacterial community composition and diversity in a smelter contaminated soil. Environ. Res. 2023, 216, 114278. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, V.K. Soil microbial biomass: A key soil driver in management of ecosystem functioning. Sci. Total Environ. 2018, 634, 497–500. [Google Scholar] [CrossRef]
- Campos, P.; Miller, A.Z.; Prats, S.A.; Knicker, H.; Hagemann, N.; De la Rosa, J.M. Biochar amendment increases bacterial diversity and vegetation cover in trace element-polluted soils: A long-term field experiment. Soil Biol. Biochem. 2020, 150, 108014. [Google Scholar] [CrossRef]
- Wang, M.; Yu, X.; Weng, X.; Zeng, X.; Li, M.; Sui, X. Meta-analysis of the effects of biochar application on the diversity of soil bacteria and fungi. Microorganisms 2023, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, H.; Delgado-Baquerizo, M.; Gundale, M.J.; Zou, X.; Ruan, H. Global meta-analysis reveals positive effects of biochar on soil microbial diversity. Geoderma 2023, 436, 116528. [Google Scholar] [CrossRef]
- Hayward, A.C.; Fegan, N.; Fegan, M.; Stirling, G.R. Stenotrophomonas and Lysobacter: Ubiquitous plant-associated gamma-proteobacteria of developing significance in applied microbiology. J. Appl. Microbiol. 2010, 108, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, J.; Xu, N.; He, T.; Meng, J.; Liu, Z. Urea hydrolysis in different farmland soils as affected by long-term biochar application. Front. Environ. Sci. 2022, 10, 1304. [Google Scholar] [CrossRef]
- Mobley, H.L.T.; Hausinger, R.P. Microbial ureases: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [CrossRef]
- Diquattro, S.; Garau, G.; Garau, M.; Lauro, G.P.; Pinna, M.V.; Castaldi, P. Effect of municipal solid waste compost on antimony mobility, phytotoxicity and bioavailability in polluted soils. Soil Syst. 2021, 5, 60. [Google Scholar] [CrossRef]
- Klik, B.; Holatko, J.; Jaskulska, I.; Gusiatin, M.Z.; Hammerschmiedt, T.; Brtnicky, M.; Liniauskienė, E.; Baltazar, T.; Jaskulski, D.; Kintl, A.; et al. Bentonite as a functional material enhancing phytostabilization of post-industrial contaminated soils with heavy metals. Materials 2022, 15, 8331. [Google Scholar] [CrossRef]
- Sladkovska, T.; Wolski, K.; Bujak, H.; Radkowski, A.; Sobol, Ł. A review of research on the use of selected grass species in removal of heavy metals. Agronomy 2022, 12, 2587. [Google Scholar] [CrossRef]
- Willscher, S.; Mirgorodsky, D.; Jablonski, L.; Ollivier, D.; Merten, D.; Büchel, G.; Wittig, J.; Werner, P. Field scale phytoremediation experiments on a heavy metal and uranium contaminated site, and further utilization of the plant residues. Hydrometallurgy 2013, 131–132, 46–53. [Google Scholar] [CrossRef]
- Doss, B.D.; Evans, C.E.; Turner, J.L. Influence of subsoil acidity on tomato yield and fruit size. J. Amer. Soc. Hort. Sci. 1977, 102, 643–645. [Google Scholar] [CrossRef]








| Physico-Chemical Characteristics | C Soil | 2.5-Bio | 5.0-Bio |
|---|---|---|---|
| Texture | Sandy loam | - | - |
| pH | 5.74 ± 0.02 a | 6.35 ± 0.01 b | 6.58 ± 0.01 c |
| CE (µS cm−1) | 376 ± 9 a | 325 ± 5 b | 333 ± 4 b |
| Organic matter (g kg−1) | 32.67 ± 0.58 a | 34.67 ± 0.58 b | 36.33 ± 0.58 c |
| Total N (g kg−1) | 1.00 ± 0.0 a | 1.00 ± 0.0 a | 0.91 ± 0.0 b |
| P Olsen (mg kg−1) | 31.53 ± 1.22 a | 32.43 ± 0.98 a | 35.73 ± 0.47 b |
| CEC (cmol(+) kg−1) | 24.36 ± 0.02 a | 24.55 ± 0.08 b | 25.15 ± 0.42 c |
| DOC (mg kg−1) | 13.79 ± 0.43 b | 12.51 ± 0.26 a | 12.04 ±0.12 a |
| Pb (mg kg−1) | 10.625 ± 2058 a | 10.238 ± 372 a | 10.064 ± 141 a |
| Cd (mg kg−1) | 28.3 ± 0.4 a | 27.2 ± 1.05 a | 27.1 ± 1.07 a |
| Zn (mg kg−1) | 3407 ± 140 a | 3291 ± 241 a | 3323 ± 120 a |
| Triticale | ||||||
| Pb Uptake (mg kg−1) | Cd Uptake (mg kg−1) | Zn Uptake (mg kg−1) | ||||
| Shoots | Roots | Shoots | Roots | Shoots | Roots | |
| C soil | 39.2 ± 0.3 b | 434.8 ± 21.5 a | 5.7 ± 0.1 b | 30.3 ± 1.1 a | 631.1 ± 25.8 a | 1596.3 ± 8.3 c |
| 2.5-Bio | 38.3 ± 0.5 a | 593.7 ± 2.9 b | 5.1 ± 0.1 a | 61.6 ± 1.1 c | 733.2 ± 5.0 c | 1307.4 ± 19.7 b |
| 5.0-Bio | 53.3 ± 0.2 c | 456.8 ± 25.3 ab | 7.9 ± 0.1 c | 52.6 ± 0.7 b | 683.4 ± 10.1 b | 928.0 ± 6.7 a |
| Tomato | ||||||
| Pb uptake (mg kg−1) | Cd uptake (mg kg−1) | Zn uptake (mg kg−1) | ||||
| Shoots | Roots | Shoots | Roots | Shoots | Roots | |
| C soil | 514.7 ± 22.8 c | ND | 17.7 ± 0.6 b | ND | 2843.1 ± 22.3 c | ND |
| 2.5-Bio | 89.2 ± 7.4 a | 695.4 ± 20.3 b | 15.3 ± 1.1 a | 133.5 ± 2.1 b | 879.1 ± 86.5 b | 4859.2 ± 28.3 b |
| 5.0-Bio | 115.5 ± 9.2 b | 616.8 ± 17.0 a | 24.9 ±0.2 c | 104.2 ± 1.7 a | 680.3 ± 9.5 a | 3101.2 ± 28.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garau, M.; Castaldi, P.; Pinna, M.V.; Diquattro, S.; Cesarani, A.; Mangia, N.P.; Vasileiadis, S.; Garau, G. Sustainable Restoration of Soil Functionality in PTE-Affected Environments: Biochar Impact on Soil Chemistry, Microbiology, Biochemistry, and Plant Growth. Soil Syst. 2023, 7, 96. https://doi.org/10.3390/soilsystems7040096
Garau M, Castaldi P, Pinna MV, Diquattro S, Cesarani A, Mangia NP, Vasileiadis S, Garau G. Sustainable Restoration of Soil Functionality in PTE-Affected Environments: Biochar Impact on Soil Chemistry, Microbiology, Biochemistry, and Plant Growth. Soil Systems. 2023; 7(4):96. https://doi.org/10.3390/soilsystems7040096
Chicago/Turabian StyleGarau, Matteo, Paola Castaldi, Maria Vittoria Pinna, Stefania Diquattro, Alberto Cesarani, Nicoletta P. Mangia, Sotirios Vasileiadis, and Giovanni Garau. 2023. "Sustainable Restoration of Soil Functionality in PTE-Affected Environments: Biochar Impact on Soil Chemistry, Microbiology, Biochemistry, and Plant Growth" Soil Systems 7, no. 4: 96. https://doi.org/10.3390/soilsystems7040096
APA StyleGarau, M., Castaldi, P., Pinna, M. V., Diquattro, S., Cesarani, A., Mangia, N. P., Vasileiadis, S., & Garau, G. (2023). Sustainable Restoration of Soil Functionality in PTE-Affected Environments: Biochar Impact on Soil Chemistry, Microbiology, Biochemistry, and Plant Growth. Soil Systems, 7(4), 96. https://doi.org/10.3390/soilsystems7040096