Clean Technologies for Production of Valuable Fractions from Sardine Cooking Wastewaters: An Integrated Process of Flocculation and Reverse Osmosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
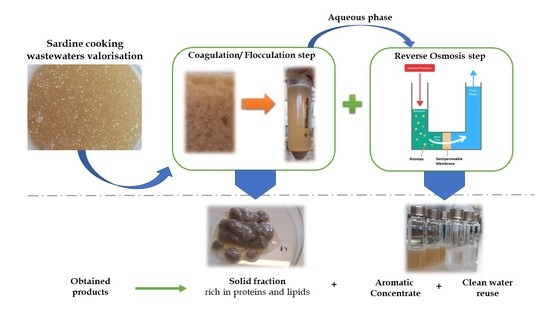
2.2. Experimental Procedure
- ▪
- In the feed preparation step, a study was performed encompassing the effect of adding the acorn extract to the sardine cooking wastewater on the chemical characteristics of the aroma profile (aiming for minimised oxidation of aromas, namely aldehydes, and minimised formation of sulphur compounds off-flavours). The feed preparation with the intended composition was selected.
- ▪
- In the coagulation–flocculation step, studies were conducted aiming to determine the effect of the concentration of the coagulant and the effects of the type and concentration of the flocculant on the chemical composition of the supernatant and solid fraction (aiming at a clarification of the supernatant by maximised proteins′ and lipids′ recovery in the solid fraction). Twelve coagulant/flocculant combinations were evaluated.
- ▪
- In the reverse osmosis step, studies were performed aiming to assess the effect of the feed source (aqueous fractions from each pre-treatment), when processed by reverse osmosis, on the composition of the concentrates (in terms of aromas) and of the permeates (in terms of COD, related to the organic load). The impact of the pre-treatment process on the membrane performance (membrane permeance) was also evaluated. For the four best coagulant/flocculant combinations, reverse osmosis experiments were performed for the selection of the combination of coagulant and flocculant concentrations, reaching the same final volumetric concentration factor of 3.
2.2.1. Coagulation/Flocculation Pre-Treatment
2.2.2. Reverse Osmosis
2.3. Analytical Methods
2.3.1. Chemical Oxygen Demand (COD) Measurement
2.3.2. Total Protein Content
2.3.3. Total Lipid Content
2.3.4. SPME/GC-MS
3. Results
3.1. Characterisation of Sardine Cooking Wastewater
Effect of the Antioxidant Extract of Acorn
3.2. Pre-Treatment: Coagulation/Flocculation Process Pre-Treatment Selection Using Different Combinations of Coagulant and Flocculant
- ▪
- Characterisation of protein and lipid recovery of the fractions obtained with the application of chitosan, carrageenan and alginate used individually
- ▪
- Characterisation of protein and lipid content of the fractions, for selection of the best combination of coagulant and flocculant and their concentrations
- ▪
- Characterisation of aroma content in the fractions obtained, for selection of the best combination of coagulant and flocculant
- ▪
- Sterilisation of the solid fractions and characterisation of their aroma content
- ▪
- Characterisation of the supernatant obtained from the four coagulant/flocculant selected combinations
3.3. Aroma Recovery by Reverse Osmosis: Selection of the Best Coagulant/Flocculant Combination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omil, F.; García-Sandá, E.; Méndez, R.; Lema, J.M. Clean technologies for wastewater management in seafood canning industries. In Technological Choices for Sustainability; Springer: Berlin/Heidelberg, Germany, 2014; pp. 103–125. [Google Scholar] [CrossRef]
- Forghani, B.; Sørensen, A.D.M.; Johannesson, J.; Svendsen, T.C.; Undeland, I. Flocculation and Flotation to Recover Protein-Enriched Biomasses from Shrimp Boiling and Peeling Process Waters: A Case Study. ACS Sustain. Chem. Eng. 2020, 8, 9660–9668. [Google Scholar] [CrossRef]
- INE (Instituto National de Estatística). Estatísticas da Pesca 2011; Instituto Nacional de Estatística: Lisbon, Portugal, 2012; ISBN 9789892501567. [Google Scholar]
- Almeida, C.; Vaz, S.; Ziegler, F. Environmental Life Cycle Assessment of a Canned Sardine Product from Portugal. J. Ind. Ecol. 2015, 19, 607–617. [Google Scholar] [CrossRef]
- Cristóvão, R.; Botelho, C.; Martins, R.; Boaventura, R. Pollution prevention and wastewater treatment in fish canning industries of Northern Portugal. Int. Proc. Chem. Biol. Environ. Eng. 2012, 32, 12–16. [Google Scholar] [CrossRef]
- Venugopal, V.; Sasidharan, A. Seafood industry effluents: Environmental hazards, treatment and resource recovery. J. Environ. Chem. Eng. 2021, 9, 104758. [Google Scholar] [CrossRef]
- Cristóvão, R.O.; Botelho, C.M.; Martins, R.J.E.; Loureiro, J.M.; Boaventura, R.A.R. Fish canning industry wastewater treatment for water reuse—A case Study. J. Clean. Prod. 2015, 87, 603–612. [Google Scholar] [CrossRef]
- Hung, Y.-T.; Show, K.-Y.; Tay, J.-H. Seafood Processing Wastewater Treatment. In Handbook of Industrial and Hazardous Wastes Treatment; CRC Press: Boca Raton, FL, USA, 2005; pp. 706–749. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Bourseau, P.; Massé, A.; Cros, S.; Vandanjon, L.; Jaouen, P. Recovery of aroma compounds from seafood cooking juices by membrane processes. J. Food Eng. 2014, 128, 157–166. [Google Scholar] [CrossRef]
- Vandanjon, L.; Cros, S.; Jaouen, P.; Quéméneur, F.; Bourseau, P. Recovery by nanofiltration and reverse osmosis of marine flavours from seafood cooking waters. Desalination 2002, 144, 379–385. [Google Scholar] [CrossRef]
- Cai, Y.H.; Galili, N.; Gelman, Y.; Herzberg, M.; Gilron, J.; Mohseni, A.; Kube, M.; Fan, L.; Roddick, F.A. Treatment of wastewater reverse osmosis concentrate using alginate-immobilised microalgae: Integrated impact of solution conditions on algal bead performance. J. Memb. Sci. 2021, 623, 119054. [Google Scholar] [CrossRef]
- Arias-Lizárraga, D.M.; Méndez-Gomez, E. Remoción de sólidos en aguas residualesde la industria harinera de pescado empleando biopolímeros. Tecnol. Ciencias Del Agua 2014, 5, 115–123. [Google Scholar]
- Venugopal, V. Valorization of Seafood Processing Discards: Bioconversion and Bio-Refinery Approaches. Front. Sustain. Food Syst. 2021, 5, 611835. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation-Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Tremblay, A.; Corcuff, R.; Goulet, C.; Godefroy, S.B.; Doyen, A.; Beaulieu, L. Valorization of American lobster (Homarus americanus) cooking waters: Preparation and characterization of a food ingredient. J. Food Process. Preserv. 2021, 45, e15665. [Google Scholar] [CrossRef]
- Tremblay, A.; Corcuff, R.; Goulet, C.; Godefroy, S.B.; Doyen, A.; Beaulieu, L. Valorization of snow crab (Chionoecetes opilio) cooking effluents for food applications. J. Sci. Food Agric. 2020, 100, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.; Mulder, J. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; Volume 148, ISBN 079234247X. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro- and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative Study on Total Lipid Determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and Modified Bligh & Dyer Extraction Methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar] [CrossRef]
- Ganeko, N.; Shoda, M.; Hirohara, I.; Bhadra, A.; Ishida, T.; Matsuda, H.; Takamura, H.; Matoba, T. Analysis of volatile flavor compounds of sardine (Sardinops melanostica) by solid phase microextraction. J. Food Sci. 2008, 73, S83–S88. [Google Scholar] [CrossRef]
- Mansur, M.A.; Bhadra, A.; Takamura, H.; Matoba, T. Volatile flavor compounds of some sea fish and prawn species. Fish. Sci. 2003, 69, 864–866. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.; Choi, A.; Cho, I.H.; Kim, Y.; Korea, S. Changes in fatty acids and volatile components in mackerel by broiling. Eur. J. Lipid Sci. Technol. 2011, 113, 1481–1490. [Google Scholar] [CrossRef]
- Martínez, R.; Sanz, M.T.; Beltrán, S. Concentration by pervaporation of representative brown crab volatile compounds from dilute model solutions. J. Food Eng. 2011, 105, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Nimesha, S.; Hewawasam, C.; Jayasanka, D.J.; Murakami, Y.; Araki, N.; Maharjan, N. Effectiveness of natural coagulants in water and wastewater treatment. Glob. J. Environ. Sci. Manag. 2022, 8, 101–116. [Google Scholar] [CrossRef]
- Jouenne, E.; Crouzet, J. Effect of pH on retention of aroma compounds by β-lactoglobulin. J. Agric. Food Chem. 2000, 48, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.R.; Shahbaz, M. Mussel protein recovery using dissolved air flotation. J. Food Eng. 1986, 5, 135–151. [Google Scholar] [CrossRef]
- Wibowo, S.; Velazquez, G.; Savant, V.; Torres, J.A. Effect of chitosan type on protein and water recovery efficiency from surimi wash water treated with chitosan-alginate complexes. Bioresour. Technol. 2007, 98, 539–545. [Google Scholar] [CrossRef]
- Rogacheva, S.; Espinosa-Diaz, M.A.; Voilley, A. Transfer of aroma compounds in water-lipid systems: Binding tendency of β-lactoglobulin. J. Agric. Food Chem. 1999, 47, 259–263. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, T.; Fu, X.; Zhu, C.; Mou, H. Partially degraded chitosan-based flocculation to achieve effective deodorization of oyster (Crassostrea gigas) hydrolysates. Carbohydr. Polym. 2020, 234, 115948. [Google Scholar] [CrossRef]
- Hirano, S.; Itakura, C.; Seino, H.; Akiyama, Y.; Nonaka, I.; Kanbara, N.; Kawakami, T. Chitosan as an Ingredient for Domestic Animal Feeds. J. Agric. Food Chem. 1990, 38, 1214–1217. [Google Scholar] [CrossRef]
- Peinado, I.; Miles, W.; Koutsidis, G. Odour characteristics of seafood flavour formulations produced with fish by-products incorporating EPA, DHA and fish oil. Food Chem. 2016, 212, 612–619. [Google Scholar] [CrossRef]
- Destani, F.; Naccarato, A.; Tagarelli, A.; Cassano, A. Recovery of aromatics from orange juice evaporator condensate streams by reverse osmosis. Membranes 2020, 10, 92. [Google Scholar] [CrossRef]
- Pozderović, A.; Popović, K.; Pichler, A.; Jakobek, L. Influencia de los parámetros de procesamiento en el flujo de permeación y la retención de compuestos aromáticos y fenólicos en el zumo concentrado de arándano silvestre mediante ósmosis inversa. CYTA-J. Food 2016, 14, 382–390. [Google Scholar] [CrossRef]
- Xie, Z.; Nagaraja, N.; Skillman, L.; Li, D.; Ho, G. Comparison of polysaccharide fouling in forward osmosis and reverse osmosis separations. Desalination 2017, 402, 174–184. [Google Scholar] [CrossRef]













| Parameter | |
|---|---|
| pH | 6.5 ± 0.1 |
| COD (mg/L) | 28,080 ± 100 |
| TSS (mg/L) | 40,734.64 ± 2520.36 |
| Total protein content (mg/mL) | 25.38 ± 1.95 |
| Total lipids content (%) | 28.13 ± 2.84 |
| Aroma Compounds | Area Ratio (%) * | Concentration (ppm) * |
|---|---|---|
| Aldehydes | ||
| Hexanal | 9.31% | |
| Heptanal | 2.15% | 0.006 |
| 2-Hexenal, (E)- | 4.36% | |
| Octanal | 1.28% | |
| Nonanal | 5.94% | |
| 2-Octenal, (E)- | 2.99% | |
| 2,4-Heptadienal, (E,E)- | 6.58% | |
| 2-Nonenal, (E)- | 1.63% | 0.011 |
| 2,6-Nonadienal, (E,Z)- | 6.90% | 0.044 |
| 2-Decenal, (E)- | 0.90% | |
| Alcohols | ||
| 1-Penten-3-ol | 6.02% | 0.100 |
| 1-Octen-3-ol | 13.69% | 0.008 |
| (5Z)-Octa-1,5-dien-3-ol | 6.50% | |
| 2-Ethylhexanol | 0.57% | |
| 1-Octanol | 44.49% | |
| Sulphur compounds | ||
| Trans-2-(2-Pentenyl)furan | 3.52% | |
| Ketones | ||
| 2-Nonanone | 3.38% | 0.001 |
| 3,5-Octadien-2-one | 8.47% | |
| (3E,5E)-3,5-octadien-2-one | 8.49% | |
| 2-Undecanone | 0.60% | |
| Acids | ||
| Hexanoic acid | 6.71% | |
| Polysaccharide | Fraction | Weight Proportion (%) | Protein Recovery in Solid Fraction (%) | Lipid Recovery in Solid Fraction (%) |
|---|---|---|---|---|
| Chitosan (100 mg/L) | Solid F. | 4.60 | 35.97 ± 2.1 | 56.02 ± 0.5 |
| Supernatant | 95.40 | |||
| Carrageenan (10 mg/L) | Solid F. | 4.35 | 41.77 ± 0.3 | 54.50 ± 4.0 |
| Supernatant | 95.65 | |||
| Alginate (10 mg/L) | Solid F. | 4.46 | 42.32 ± 0.8 | 40.22 ± 1.5 |
| Supernatant | 95.54 |
| Treatment (mg/L) | Sample | Weight Proportion (%) | Protein Content (mg/mL) | Lipid Content (g/100 g) | Protein Recovery in Solid Fraction (%) | Lipid Recovery in Solid Fraction (%) |
|---|---|---|---|---|---|---|
| 100/10 * | Solid F. | 4.66 | 30.33 ± 2.84 | 25.26 ± 0.92 | 79 ± 1 | 64 ± 4 |
| Supernatant | 95.34 | 5.44 ± 0.30 | 10.16 ± 1.73 | |||
| 200/20 | Solid F. | 10.41 | 21.19 ± 1.59 | 20.02 ± 2.50 | 77 ± 2 | 68 ± 3 |
| Supernatant | 89.60 | 5.92 ± 0.55 | 9.08 ± 1.08 | |||
| —300/30 | Solid F. | 6.34 | 24.70 ± 0.29 | 26.11 ± 0.44 | 73 ± 1 | 55 ± 2 |
| Supernatant | 93.66 | 6.80 ± 0.29 | 10.63 ± 3.45 | |||
| 400/40 | Solid F. | 5.38 | 27.55 ± 1.86 | 18.99 ± 2.26 | 75 ± 1 | 65 ± 7 |
| Supernatant | 94.62 | 6.47 ± 0.13 | 9.35 ± 2.17 | |||
| 500/50 | Solid F. | 10.39 | 16.83 ± 1.38 | 7.06 ± 1.21 | 73 ± 2 | 72 ± 4 |
| Supernatant | 89.61 | 6.87 ± 0.39 | 7.77 ± 1.51 | |||
| 600/60 | Solid F. | 8.16 | 25.12 ± 2.30 | 16.78 ± 1.44 | 71 ± 2 | 82 ± 1 |
| Supernatant | 91.84 | 7.28 ± 0.57 | 5.13 ± 0.06 |
| Treatment (mg/L) | Sample | Weight Proportion (%) | Protein Content (mg/mL) | Lipid Content(g/100 g) | Protein Recovery in Solid Fraction (%) | Lipid Recovery in Solid Fraction (%) |
|---|---|---|---|---|---|---|
| 100/10 * | Solid F. | 8.56 | 22.49 ± 1.66 | 10.98 ± 1.37 | 78 ± 3 | 46 ± 2 |
| Supernatant | 91.44 | 5.64 ± 0.76 | 11.37 ± 0.76 | |||
| 200/20 | Solid F. | 6.07 | 19.10 ± 0.27 | 7.44 ± 0.86 | 43 ± 6 | 49 ± 4 |
| Supernatant | 93.93 | 14.51 ± 1.51 | 14.40 ± 1.62 | |||
| 300/30 | Solid F. | 5.27 | 34.99 ± 1.66 | 8.97 ± 3.84 | 34 ± 4 | 38 ± 5 |
| Supernatant | 94.73 | 16.86 ± 0.95 | 17.52 ± 2.24 | |||
| 400/40 | Solid F. | 4.84 | 31.08 ± 0.59 | 24.65 ± 0.56 | 35 ± 2 | 42 ± 2 |
| Supernatant | 95.16 | 16.62 ± 0.71 | 16.25 ± 0.63 | |||
| 500/50 | Solid F. | 4.46 | 49.37 ± 1.36 | 39.61 ± 1.51 | 41 ± 2 | 34 ± 1 |
| Supernatant | 95.54 | 15.07 ± 0.51 | 14.36 ± 0.60 | |||
| 600/60 | Solid F. | 5.72 | 46.78 ± 1.00 | 24.65 ± 0.11 | 24 ± 3 | 44 ± 4 |
| Supernatant | 94.28 | 19.27 ± 0.91 | 15.84 ± 3.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, M.J.; Grosjean, O.; Pintado, M.; Brazinha, C.; Crespo, J. Clean Technologies for Production of Valuable Fractions from Sardine Cooking Wastewaters: An Integrated Process of Flocculation and Reverse Osmosis. Clean Technol. 2022, 4, 276-295. https://doi.org/10.3390/cleantechnol4020016
Pereira MJ, Grosjean O, Pintado M, Brazinha C, Crespo J. Clean Technologies for Production of Valuable Fractions from Sardine Cooking Wastewaters: An Integrated Process of Flocculation and Reverse Osmosis. Clean Technologies. 2022; 4(2):276-295. https://doi.org/10.3390/cleantechnol4020016
Chicago/Turabian StylePereira, Maria João, Oceane Grosjean, Manuela Pintado, Carla Brazinha, and João Crespo. 2022. "Clean Technologies for Production of Valuable Fractions from Sardine Cooking Wastewaters: An Integrated Process of Flocculation and Reverse Osmosis" Clean Technologies 4, no. 2: 276-295. https://doi.org/10.3390/cleantechnol4020016
APA StylePereira, M. J., Grosjean, O., Pintado, M., Brazinha, C., & Crespo, J. (2022). Clean Technologies for Production of Valuable Fractions from Sardine Cooking Wastewaters: An Integrated Process of Flocculation and Reverse Osmosis. Clean Technologies, 4(2), 276-295. https://doi.org/10.3390/cleantechnol4020016