The Corrosion Inhibition Performance of Eco-Friendly bis-Schiff Bases on Carbon Steel in a Hydrochloric Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Corrosion Inhibitors
2.2. Electrochemical Measurements
2.2.1. Electrochemical Impedance Spectroscopy (EIS)
2.2.2. Linear Polarization Resistance (LPR)
2.2.3. Potentiodynamic Polarization (PP)
2.3. Weight Loss Method
2.4. Surface Investigation
Scanning Electron Microscopy
2.5. Theoretical Calculations
3. Results
3.1. Synthesis
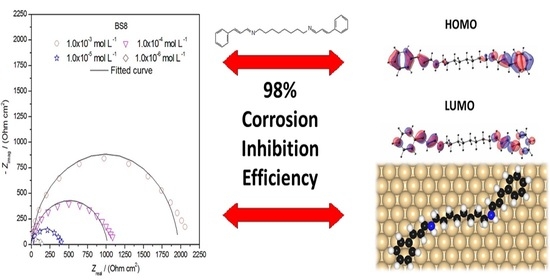
3.2. EIS
3.3. LPR
3.4. PP
3.5. Adsorption Phenomenon
3.6. Weight Loss Method
3.6.1. Immersion Time Effect
3.6.2. Effect of Temperature
3.7. Scanning Electron Microscopy (SEM)
3.8. Theoretical Studies
3.8.1. Molecular Properties of BS2, BS4 and BS8
3.8.2. Adsorption of the BS2, BS4 and BS8 Inhibitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Quraishi, M.A. Corrosion inhibition of mild steel using novel bis Schiff’s bases as corrosion inhibitors: Electrochemical and surface measurement. Measurement 2016, 8, 114. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning for ferrous and non-ferrous metals in acidic solution: A review. J. Mol. Liq. 2018, 256, 565. [Google Scholar] [CrossRef]
- Nam, N.D.; Somers, A.; Mathesh, M.; Seter, M.; Hinton, B.; Forsyth, M.; Tan, M.Y.J. The behavior of praseodymium 4-hydroxycynnamate as an inhibitor for carbon dioxide corrosion and oxygen corrosion of steel of NaCl solutions. Corros. Sci. 2014, 80, 128. [Google Scholar] [CrossRef]
- Quartarone, G.; Ronchin, L.; Vavasori, A.; Tortato, C.; Bonaldo, L. Inhibitive action of gramine towards corrosion of mild steel in deaerated 1.0 M in hydrochloric acid solutions. Corros. Sci. 2012, 64, 82. [Google Scholar] [CrossRef]
- de Souza, F.S.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642. [Google Scholar] [CrossRef]
- Singh, D.K.; Ebenso, E.E.; Singh, M.K.; Behera, D.; Udayabhanu, G.; John, R.P. Non-toxic Schiff bases as efficient corrosion inhibitors for mild steel in 1 M HCl: Electrochemical, AFM, FE-SEM and theoretical studies. J. Mol. Liq. 2018, 250, 88. [Google Scholar] [CrossRef]
- Arjomandi, J.; Moghanni-Bavil-Olvaei, H.; Parvin, M.H.; Lee, J.Y.; Ko, K.C.; Joshaghani, M.; Hamidian, K. Inhibition of corrosion of aluminum in alkaline solution by a novel azo-Schiff base: Experiment and theory. J. Alloys Compd. 2018, 746, 185. [Google Scholar] [CrossRef]
- Soltani, N.; Behpour, M.; Ghoreishi, S.M.; Naeimi, H. Corrosion inhibition of mild steel in hydrochloric acid solution by some double Schiff bases. Corros. Sci. 2010, 52, 1351. [Google Scholar] [CrossRef]
- Mallaiya, E.; Subramania, R.; Srikandan, S.S.; Gowra, S.; Rajasekaran, N.; Selvaraj, A. Electrochemical characterization of the protective film formed by the unsymmetrical Schiff’s base on the mild steel surface in acid media. Electrochim. Acta 2011, 56, 3857. [Google Scholar] [CrossRef]
- Palomar-Pardavé, M.; Romero-Romo, M.; Herrera-Hernández, H.; Abreu-Quijano, M.A.; Likhanova, N.V.; Uruchurtu, J.; Juárez-García, J.M. Influence of the alkyl chain length of 2 amino 5 alkyl 1,3,4-thiadiazole compounds on the corrosion inhibition of steel immersed in sulfuric acid solutions. Corros. Sci. 2012, 54, 231. [Google Scholar] [CrossRef]
- Shahabi, S.; Norouzi, P.; Ganiali, M.R. Theoretical and electrochemical study of carbon steel corrosion inhibition in the presence of two synthesized Schiff base inhibitors: Application of fast Fourier transform continuous cyclic voltammetry to study the adsorption behaviour. Int. J. Electrochem. Sci. 2015, 10, 2646. Available online: http://www.electrochemsci.org/papers/vol (accessed on 24 March 2022). [CrossRef]
- Liang, C.; Liu, Z.; Liang, Q.; Han, G.; Han, J.; Zhang, S.; Feng, X.Z. Synthesis of 2-aminofluorene bis Schiff base and corrosion inhibition performance for carbon steel in HCl. J. Mol. Liq. 2019, 277, 330. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L−1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Corros. Sci. 2019, 146, 134. [Google Scholar] [CrossRef]
- Jetti, V.; Pagadala, R.; Meshram, J.S.; Chopde, H.N.; Malladi, L. Zeolite-supported on-pot synthesis of bis-azetidinones under microwave irradiation. J. Heterocycl. Chem. 2013, 50, E160. [Google Scholar] [CrossRef]
- Das, S.; Das, V.K.; Saikia, L.; Thakur, A.J. Environment-friendly and solvent-free synthesis of symmetrical bis-imines under microwave irradiation. Green Chem. Lett. Rev. 2012, 5, 457. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Houk, K.N. Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous solvation free energies of neutral and ionic organic molecules. J. Chem. Theory Comput. 2005, 1, 70. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.F.; Lonie, D.C.; Vandermeersch, C.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 1. [Google Scholar] [CrossRef]
- Chemcraft–Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 14 October 2019).
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors: Part 1: An overview. Corros. Sci. 2015, 99, 1. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 4574, 747. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T. Über die zuordnung von wellenfunktionen und eigenwerten zu den einzelnen elektronen eines atoms. Physica 1934, 1, 104. [Google Scholar] [CrossRef]
- Iczkowski, R.P.; Margrave, J.L. Electronegativity. J. Am. Chem. Soc. 1961, 83, 3547. [Google Scholar] [CrossRef]
- Janak, J.F. Proof that E/ni = i in density-functional theory. Phys. Rev. B 1978, 18, 7165. [Google Scholar] [CrossRef]
- von Szentpály, L. Studies on electronegativity equalization. Part 1. Consistent diatomic partial charges. J. Mol. Struct. THEOCHEM 1991, 233, 71. [Google Scholar] [CrossRef]
- Pearson, R.G. Recent advances in the concept of hard and soft acids and bases. J. Chem. Ed. 1987, 64, 561. [Google Scholar] [CrossRef]
- Parr, R.G.; Sventpaly, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533. [Google Scholar] [CrossRef]
- Sastri, V.S.; Perumareddi, J.R. Molecular orbital theoretical studies of some organic corrosion inhibitors. Corrosion 1997, 53, 617. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion inhibitors-correlation between electronic structure and efficiency. Corrosion 2001, 57, 3. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 5th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1976. [Google Scholar]
- Spencer, M.J.S.; Hung, A.; Snook, I.K.; Yarovsky, I. Density functional theory study of the relaxation and energy of iron surfaces. Surf. Sci. 2002, 513, 389. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.J. Aromatic adsorption on metals via first-principles density functional theory. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 465, 2949–2976. [Google Scholar] [CrossRef]
- Liu, W.; Ruiz, V.G.; Zhang, G.X.; Santra, B.; Ren, X.; Scheffler, M.; Tkatchenko, A. Structure and energetics of benzene adsorbed on transition-metal surfaces: Density-functional theory with van der Waals interactions including collective substrate response. New J. Phys. 2013, 15, 053046. [Google Scholar] [CrossRef]
- Makov, G.; Payne, M.C. Periodic boundary conditions in ab initio calculations. Phys. Rev. B 1995, 51, 4014. [Google Scholar] [CrossRef]
- Mendonça, G.L.F.; Costa, S.N.; Freire, V.N.; Casciano, P.N.S.; Correia, A.N.; Lima-Neto, P. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid by amino acids using electrochemical technics allied to molecular modelling methods. Corros. Sci. 2017, 115, 41. [Google Scholar] [CrossRef]
- Carlos, M.F.L.P.; Valbon, A.; Neves, M.A.; Santos, M.R.L.; Echevarria, A. β-Enaminoesters as novel corrosion inhibitors for carbon steel in acidic medium. J. Braz. Chem. Soc. 2018, 29, 2542. [Google Scholar] [CrossRef]
- Valbon, A.; Neves, M.A.; Echevarria, A. Anticorrosive effect of PVP 40000 against AISI 1020 carbon steel in HCl. Mater. Res. 2018, 6, e20170847. [Google Scholar] [CrossRef]
- Chaitra, T.K.; Mohana, K.N.S.; Tandon, H.C. Thermodynamic, electrochemical and quantum evaluation of some triazole Schiff bases as mild steel corrosion inhibitors in acid media. J. Mol. Liq. 2015, 211, 10268. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical impedance spectroscopy of metal oxide electrodes for energy applications. ACS Appl. Energy Mater. 2020, 3, 66. [Google Scholar] [CrossRef]
- Taylor, C.D. Corrosion informatics: An integrated approach to modelling corrosion. Corros. Eng. Sci. Technol. 2015, 50, 490. [Google Scholar] [CrossRef]
- Kumar, C.B.P.; Mohana, K.N. Corrosion inhibitor efficiency and adsorption characteristics of some Schiff bases at mild steel/hydrochloric acid interface. J. Taiwan Inst. Chem. Eng. 2014, 45, 1031. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A. Bis-Schiff bases of isatin as new and environmentally benign corrosion inhibitor for mild steel. J. Ind. Eng. Chem. 2014, 20, 2819. [Google Scholar] [CrossRef]
- Erami, R.S.; Amirnar, M.; Meghdadi, S.; Talebian, M.; Farrokhpour, H.; Raeissi, K. Carboxamide derivatives as new corrosion inhibitors for mild steel protection in hydrochloric acid solution. Corros. Sci. 2019, 151, 190. [Google Scholar] [CrossRef]
- Fan, B.; Ma, Y.; Wang, M.; Hao, H.; Yang, B.; Li, J.; Sun, H. Revealing the assembly mechanism of an octadecylamine based supramolecular complex on mild steel in condensate water: Correlative experimental and theoretical studies. J. Mol. Liq. 2019, 292, 111446. [Google Scholar] [CrossRef]
- Yurt, A.; Duran, B.; Dal, H. An experimental and theoretical investigation on adsorption properties of some diphenolic Schiff bases as corrosion inhibitors at acidic solution/mild steel interface. Arab. J. Chem. 2014, 7, 732. [Google Scholar] [CrossRef]
- Biswas, A.; Mourya, P.; Mondal, D.; Pal, S.; Udayabhanu, G. Grafting effect of gum acacia on mild steel corrosion in acidic medium: Gravimetric and electrochemical study. J. Mol. Liq. 2018, 251, 470. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Lin, T.; Xie, X.; Du, G. Cassava starch-sodium allylsulfonate-acryl amide graft copolymer as an effective inhibitor of aluminum corrosion in HCl solution. J. Taiwan Inst. Chem. Eng. 2018, 86, 252. [Google Scholar] [CrossRef]
- Gerengi, H.; Mielniczek, M.; Gece, G.; Solomon, M.M. Experimental and quantum chemical evaluation of 8-hydroxyquinoline as a corrosion inhibitor for copper in 1.0 m HCl. Ind. Eng. Chem. Res. 2016, 55, 9614. [Google Scholar] [CrossRef]
- Gong, Z.; Peng, S.; Huang, X.; Gao, L. Investigation of corrosion inhibition effect of itraconazole of copper in H2SO4 at different temperatures: Combining an experimental and theoretical studies. Materials 2018, 11, 2107. [Google Scholar] [CrossRef] [PubMed]
- Ishaka, A.; Adams, F.V.; Madua, J.O.; Josepha, I.V.; Olubambi, P.A. Corrosion inhibition of mild steel in 1M hydrochloric acid using Haematostaphis barteri leaves extract. Procedia Manuf. 2019, 35, 1279. [Google Scholar] [CrossRef]
- Umorena, S.A.; Solomon, M.M.; Alib, S.A.; Dafalla, H.D.M. Synthesis, characterization, and utilization of a diallylmethylamine-based cyclopolymer for corrosion mitigation in simulated acidizing environment. Mat. Sci. Eng. C 2019, 100, 897. [Google Scholar] [CrossRef]
- Ahamad, I.; Prasad, R.; Quaraishi, M.A. Adsorption and inhibitive properties of some new Mannich bases of isatin derivatives on corrosion of mild steel in acidic media. Corros. Sci. 2010, 52, 1472. [Google Scholar] [CrossRef]
- Chugh, B.; Singh, A.K.; Thakur, S.; Pani, B.; Pandey, A.K.; Lgaz, H.; Chung, O.M.; Ebenso, E.F. An exploration about the interaction of mild steel with hydrochloric acid in the presence of N-(benzo[d]thiazole-2-yl)-1-phenylethan-1-imines. J. Phys. Chem. C 2019, 123, 22897. [Google Scholar] [CrossRef]
- Hensley, A.J.R.; Zhang, R.; Wang, Y.; McEwen, J.-S. Tailoring the adsorption of benzene on PdFe surfaces: A density functional theory study. J. Phys. Chem. C 2013, 117, 24317. [Google Scholar] [CrossRef]

















| Compound | Cinh (mol L−1) | OCP/ Ag/AgCl (mV) | Rct (Ω cm2) | n | Y0 (µOhm cm−2) | fmax (Hz) | Cdl (µF cm−2) | Ө | ηEIS ± SD (%) |
|---|---|---|---|---|---|---|---|---|---|
| Blank | - | −436 | 50 | 0.869 | 200 | 31.6 | 99 | - | - |
| BS2 | 1.0 × 10−6 | −437 | 63 | 0.847 | 205 | 31.6 | 91 | 0.206 | 21.1 ± 0.7 |
| 1.0 × 10−5 | −434 | 92 | 0.843 | 190 | 19.95 | 89 | 0.456 | 46.0 ± 0.3 | |
| 1.0 × 10−4 | −434 | 169 | 0.829 | 152 | 12.58 | 72 | 0.704 | 70.2 ± 0.6 | |
| 1.0 × 10−3 | −417 | 239 | 0.819 | 127 | 10.0 | 60 | 0.790 | 79.1 ± 0.3 | |
| BS4 | 1.0 × 10−6 | −432 | 90 | 0.828 | 197 | 19.95 | 86 | 0.444 | 44.3 ± 0.4 |
| 1.0 × 10−5 | −422 | 245 | 0.831 | 142 | 10.00 | 70 | 0.796 | 79.2 ± 0.9 | |
| 1.0 × 10−4 | −411 | 549 | 0.858 | 77 | 6.30 | 45 | 0.908 | 91.6 ± 0.5 | |
| 1.0 × 10−3 | −411 | 1043 | 0.924 | 51 | 3.98 | 40 | 0.952 | 95.4 ± 0.8 | |
| BS8 | 1.0 × 10−6 | −422 | 138 | 0.823 | 174 | 15.84 | 77 | 0.637 | 64.1 ± 0.7 |
| 1.0 × 10−5 | −411 | 410 | 0.841 | 95 | 7.94 | 51 | 0.878 | 88.1 ± 0.8 | |
| 1.0 × 10−4 | −411 | 1058 | 0.889 | 55 | 3.98 | 38 | 0.952 | 95.4 ± 0.3 | |
| 1.0 × 10−3 | −422 | 2069 | 0.930 | 39 | 2.51 | 31 | 0.976 | 98.3 ± 0.4 |
| Compound | Cinh (mol L−1) | Eoc/ (mV) | RP (Ω cm2) | Ө | ηLPR ± SD (%) |
|---|---|---|---|---|---|
| Blank | - | −441 | 52 | - | - |
| BS2 | 1.0 × 10−6 | −439 | 63 | 0.174 | 17.2 ± 0.5 |
| 1.0 × 10−5 | −436 | 97 | 0.464 | 46.1 ± 0.3 | |
| 1.0 × 10−4 | −436 | 176 | 0.704 | 70.4 ± 0.6 | |
| 1.0 × 10−3 | −420 | 244 | 0.786 | 79.0 ± 0.6 | |
| BS4 | 1.0 × 10−6 | −435 | 93 | 0.440 | 44.2 ± 0.7 |
| 1.0 × 10−5 | −425 | 325 | 0.840 | 84.1 ± 0.3 | |
| 1.0 × 10−4 | −413 | 557 | 0.906 | 91.6 ± 0.3 | |
| 1.0 × 10−3 | −413 | 1155 | 0.955 | 95.1 ± 0.7 | |
| BS8 | 1.0 × 10−6 | −424 | 149 | 0.651 | 64.3 ± 0.7 |
| 1.0 × 10−5 | −413 | 416 | 0.875 | 88.2 ± 0.9 | |
| 1.0 × 10−4 | −401 | 1118 | 0.953 | 95.4 ± 0.1 | |
| 1.0 × 10−3 | −414 | 1915 | 0.972 | 97.6 ± 0.4 |
| Compound | Cinh (mol L−1) | Ecorr vs. Ag/AgCl (mV) | jcorr (mA cm−2) | βa (mV dec−1) | −βc (mV dec−1) | Ө | ηPP ± SD (%) |
|---|---|---|---|---|---|---|---|
| Blank | - | −427 | 0.314 | 71 | 101 | - | - |
| BS2 | 1.0 × 10−6 | −427 | 0.255 | 69 | 110 | 0.188 | 19.4 ± 0.8 |
| 1.0 × 10−5 | −427 | 0.188 | 70 | 117 | 0.401 | 40.1 ± 0.5 | |
| 1.0 × 10−4 | −422 | 0.145 | 82 | 161 | 0.538 | 54.0 ± 0.5 | |
| 1.0 × 10−3 | −402 | 0.102 | 85 | 133 | 0.675 | 67.1 ± 0.2 | |
| BS4 | 1.0 × 10−6 | -425 | 0.203 | 71 | 118 | 0.354 | 35.3 ± 0.2 |
| 1.0 × 10−5 | −415 | 0.066 | 76 | 117 | 0.789 | 79.6 ± 0.6 | |
| 1.0 × 10−4 | −389 | 0.054 | 67 | 75 | 0.828 | 83.2 ± 0.5 | |
| 1.0 × 10−3 | −365 | 0.032 | 61 | 69 | 0.898 | 90.1 ± 0.5 | |
| BS8 | 1.0 × 10−6 | −413 | 0.113 | 74 | 134 | 0.640 | 64.4 ± 0.5 |
| 1.0 × 10−5 | −400 | 0.052 | 64 | 90 | 0.834 | 83.2 ± 0.9 | |
| 1.0 × 10−4 | −374 | 0.017 | 52 | 67 | 0.946 | 95.0 ± 0.1 | |
| 1.0 × 10−3 | −354 | 0.008 | 43 | 80 | 0.974 | 97.4 ± 0.8 |
| Adsorption Isotherm | Inhibitor | Correlation Coefficient | Slope (y) | 1/y |
|---|---|---|---|---|
| Langmuir | BS2 | 0.999 | 1.02 | - |
| BS4 | 0.999 | 1.04 | - | |
| BS8 | 0.999 | 1.26 | - | |
| Temkim | BS2 | 0.980 | 0.08 | - |
| BS4 | 0.880 | 0.07 | - | |
| BS8 | 0.908 | 0.04 | - | |
| Frumkim | BS2 | 0.946 | −6.25 | - |
| BS4 | 0.746 | −5.36 | - | |
| BS8 | 0.847 | −8.96 | - | |
| El-Awady | BS2 | 0.982 | 0.41 | 2.43 |
| BS4 | 0.982 | 0.45 | 2.22 | |
| BS8 | 0.987 | 0.45 | 2.22 |
| Inhibitor | Kads | ΔG0ads (kJ mol−1) |
|---|---|---|
| BS2 | 3.70 × 104 | −36.02 |
| BS4 | 12.01 × 105 | −44.63 |
| BS8 | 47.17 × 105 | −48.03 |
| Inhibitor (mol L−1) | 3 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|---|
| Wcorr (mg cm−2 h−1) | η ± SD (%) | Wcorr (mg cm−2 h−1) | η ± SD (%) | Wcorr (mg cm−2 h−1) | η ± SD (%) | ||
| Blank | - | 1.358 | - | 1.116 | - | 1.004 | - |
| BS2 | 1.0 × 10−5 | 0.377 | 72.2 ± 0.1 | 0.340 | 69.1 ± 0.01 | 0.350 | 65.0 ± 0.04 |
| 1.0 × 10−4 | 0.147 | 89.4 ± 0.5 | 0.108 | 90.3 ± 0.03 | 0.078 | 92.5 ± 0.03 | |
| 1.0 × 10−3 | 0.129 | 90.4 ± 0.9 | 0.088 | 92.3 ± 0.05 | 0.036 | 96.2 ± 0.04 | |
| BS4 | 1.0 × 10−5 | 0.223 | 83.1 ± 0.3 | 0.200 | 82.0 ± 0.60 | 0.186 | 81.1 ± 0.02 |
| 1.0 × 10−4 | 0.095 | 93.0 ± 0.2 | 0.054 | 95.4 ± 0.02 | 0.043 | 96.2 ± 0.04 | |
| 1.0 × 10−3 | 0.045 | 97.2 ± 0.2 | 0.030 | 97.6 ± 0.02 | 0.032 | 97.4 ± 0.05 | |
| BS8 | 1.0 × 10−5 | 0.261 | 81.6 ± 0.8 | 0.150 | 86.2 ± 0.02 | 0.176 | 82.0 ± 0.10 |
| 1.0 × 10−4 | 0.088 | 93.2 ± 0.2 | 0.042 | 96.1 ± 0.06 | 0.043 | 96.1 ± 0.06 | |
| 1.0 × 10−3 | 0.038 | 97.1 ± 0.1 | 0.023 | 98.2 ± 0.04 | 0.020 | 98.3 ± 0.09 | |
| Inhibitor (1.0 × 10−3 mol L−1) | 30 °C | 40 °C | 50 °C | 60 °C | ||||
|---|---|---|---|---|---|---|---|---|
| Wcorr (mg cm−2 h−1) | η ± SD (%) | Wcorr (mg cm−2 h−1) | η ± SD (%) | Wcorr (mg cm−2 h−1) | η ± SD (%) | Wcorr (mg cm−2 h−1) | η ± SD (%) | |
| Blank | 1.358 | - | 2.285 | - | 3.485 | - | 4.942 | - |
| BS2 | 0.121 | 90.1 ± 0.10 | 0.228 | 90.2 ± 0.30 | 0.361 | 89.3 ± 0.05 | 0.647 | 87.2 ± 0.08 |
| BS4 | 0.045 | 97.4 ± 0.20 | 0.091 | 96.3 ± 0.1 | 0.187 | 95.4 ± 0.10 | 0.360 | 93.4 ± 0.10 |
| BS8 | 0.038 | 97.2 ± 0.10 | 0.080 | 96.0 ± 0.04 | 0.150 | 96.1 ± 0.10 | 0.310 | 94.2 ± 0.05 |
| Inhibitor (1.0 × 10−3 mol L−1) | Ea (kJ mol−1) | ΔH≠ (kJ mol−1) | ΔS≠ (J K−1 mol−1) |
|---|---|---|---|
| Blank | 36.13 | 33.45 | −252.96 |
| BS2 | 46.08 | 43.80 | −239.13 |
| BS4 | 58.03 | 55.32 | −209.38 |
| BS8 | 57.50 | 54.99 | −211.77 |
| Reactivity Molecular Descriptor | BS2 | BS4 | BS8 |
|---|---|---|---|
| HOMO (eV) | −6.9596 | −6.9261 | −6.9104 |
| LUMO (eV) | −3.2044 | −3.0325 | −2.9781 |
| Energy Gap (eV) | 3.7552 | 3.8936 | 3.9323 |
| Ionization Potential (eV) | 6.9596 | 6.9261 | 6.9104 |
| Electron Affinity (eV) | 3.2044 | 3.0325 | 2.9781 |
| Electronegativity (eV) | 5.082 | 4.9793 | 4.94425 |
| Global Hardness (eV) | 1.8776 | 1.9468 | 1.96615 |
| Global Softness (eV−1) | 0.532595 | 0.513663 | 0.508608 |
| Electrophilicity index (eV) | 3.438795 | 3.183869 | 3.108309 |
| Nucleophilicity index (eV−1) | 0.2908 | 0.314083 | 0.321718 |
| Fraction of electrons transferred | 0.510758 | 0.51898 | 0.522786 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valbon, A.; Xavier, N.F., Jr.; Carlos, M.F.L.P.; Bauerfeldt, G.F.; Almeida-Neto, F.W.Q.; de Lima-Neto, P.; Neves, M.A.; Rodrigues-Santos, C.E.; Echevarria, A. The Corrosion Inhibition Performance of Eco-Friendly bis-Schiff Bases on Carbon Steel in a Hydrochloric Solution. Surfaces 2023, 6, 509-532. https://doi.org/10.3390/surfaces6040034
Valbon A, Xavier NF Jr., Carlos MFLP, Bauerfeldt GF, Almeida-Neto FWQ, de Lima-Neto P, Neves MA, Rodrigues-Santos CE, Echevarria A. The Corrosion Inhibition Performance of Eco-Friendly bis-Schiff Bases on Carbon Steel in a Hydrochloric Solution. Surfaces. 2023; 6(4):509-532. https://doi.org/10.3390/surfaces6040034
Chicago/Turabian StyleValbon, Arthur, Neubi F. Xavier, Jr., Mariana F. L. P. Carlos, Glauco F. Bauerfeldt, Francisco W. Q. Almeida-Neto, Pedro de Lima-Neto, Marcelo A. Neves, Cláudio E. Rodrigues-Santos, and Aurea Echevarria. 2023. "The Corrosion Inhibition Performance of Eco-Friendly bis-Schiff Bases on Carbon Steel in a Hydrochloric Solution" Surfaces 6, no. 4: 509-532. https://doi.org/10.3390/surfaces6040034
APA StyleValbon, A., Xavier, N. F., Jr., Carlos, M. F. L. P., Bauerfeldt, G. F., Almeida-Neto, F. W. Q., de Lima-Neto, P., Neves, M. A., Rodrigues-Santos, C. E., & Echevarria, A. (2023). The Corrosion Inhibition Performance of Eco-Friendly bis-Schiff Bases on Carbon Steel in a Hydrochloric Solution. Surfaces, 6(4), 509-532. https://doi.org/10.3390/surfaces6040034