Microstructure and Properties of Thin-Film Submicrostructures Obtained by Rapid Thermal Treatment of Nickel Films on Silicon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Application and Processing of Nickel Films
2.2. Research Methods
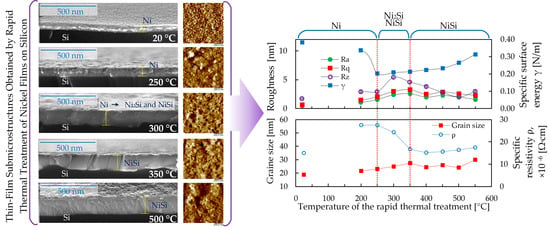
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizwan, M.N.; Kalyar, M.A.; Bell, C.; Anwar-Ul-Haq, M.; Makhdoom, A.R. Nickel thin films grown by pulsed laser deposition: Influence of substrate and substrate temperature. Dig. J. Nanometer. Bios. 2020, 15, 1141–1151. [Google Scholar] [CrossRef]
- Dzhumaliev, A.S.; Nikulin, Y.V.; Filimonov, Y.A. Formation of textured Ni (200) and Ni (111) films by magnetron sputtering. Tech. Phys. 2016, 61, 924–928. [Google Scholar] [CrossRef]
- Kuchuk, A.V.; Gołaszewska, K.; Kladko, V.P.; Guziewicz, M.; Wzorek, M.; Kamińska, E.; Piotrowska, A. The formation mechanism of Ni-based ohmic contacts to 4H-n-SiC. Mater. Sci. Forum 2012, 717, 833–836. [Google Scholar] [CrossRef]
- Testov, O.A.; Komlev, A.E.; Gareev, K.G.; Khmelnitskiy, I.K.; Luchinin, V.V.; Sevost’yanov, E.N.; Testov, I.O. Providing a specified level of electromagnetic shielding with nickel thin films formed by DC magnetron sputtering. Coatings 2021, 11, 1455. [Google Scholar] [CrossRef]
- Mangelinck, D.; Gas, P.; Grob, A.; Pichaud, B.; Thomas, O. Formation of Ni silicide from Ni (Au) films on (111) Si. J. Appl. Phys. 1996, 79, 4078–4086. [Google Scholar] [CrossRef]
- Zhao, F.F.; Zheng, J.Z.; Shen, Z.X.; Osipowicz, T.; Gao, W.Z.; Chan, L.H. Thermal stability study of NiSi and NiSi2 thin films. Microelectron. Eng. 2004, 71, 104–111. [Google Scholar] [CrossRef]
- Okubo, K.; Tsuchiya, Y.; Nakatsuka, O.; Sakai, A.; Zaima, S.; Yasuda, Y. Influence of structural variation of Ni silicide thin films on electrical property for contact materials. Jpn. J. Appl. Phys. 2004, 43, 1896. [Google Scholar] [CrossRef]
- Liu, C.M.; Liu, W.L.; Hsieh, S.H.; Tsai, T.K.; Chen, W.J. Interfacial reactions of electroless nickel thin films on silicon. Appl. Surf. Sci. 2005, 243, 259–264. [Google Scholar] [CrossRef]
- Waidmann, S.; Kahlert, V.; Streck, C.; Press, P.; Kammler, T.; Dittmar, K.; Rinderknecht, J. Tuning nickel silicide properties using a lamp based RTA, a heat conduction based RTA or a furnace anneal. Microelectron. Eng. 2006, 83, 11–12, 2282–2286. [Google Scholar] [CrossRef]
- Colgan, E.G.; Mäenpää, M.; Finetti, M.; Nicolet, M.A. Electrical characteristics of thin Ni2Si, NiSi, and NiSi2 layers grown on silicon. J. Electron. Mater. 1983, 12, 413–422. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Tobioka, A.; Nakatsuka, O.; Ikeda, H.; Sakai, A.; Zaima, S.; Yasuda, Y. Electrical properties and solid-phase reactions in Ni/Si (100) contacts. Jpn. J. Appl. Phys. 2002, 41, 2450. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Sriram, S.; Perova, T.S.; Ermakov, V.; Thorogood, G.J.; Short, K.T.; Holland, A.S. In situ micro-Raman analysis and X-ray diffraction of nickel silicide thin films on silicon. Micron 2009, 40, 89–93. [Google Scholar] [CrossRef]
- Adusumilli, P.; Seidman, D.N.; Murray, C.E. Silicide-phase evolution and platinum redistribution during silicidation of Ni0.95Pt0.05/Si(100) specimens. J. Appl. Phys. 2012, 112, 064307. [Google Scholar] [CrossRef]
- Utlu, G.; Artunc, N.; Selvi, S. Temperature and thickness dependence of the grain boundary scattering in the Ni–Si silicide films formed on silicon substrate at 500 °C by RTA. Mater. Chem. Phys. 2012, 132, 421–430. [Google Scholar] [CrossRef]
- Solovjov, J.A.; Pilipenko, V.A. Effect of Rapid Thermal Treatment Temperature on Electrophysical Properties of Nickel Films on Silicon. Doklady BGUIR 2020, 18, 81–88. [Google Scholar] [CrossRef]
- Pascu, R.; Romanitan, C. Phase transition of nickel silicide compounds and their electrical properties. J. Mater. Sci. Mater. Electron. 2021, 32, 16811–16823. [Google Scholar] [CrossRef]
- Pilipenko, V.A.; Solovjov, J.A.; Gaiduk, P.I. Nickel silicide formation with rapid thermal treatment in the heat balance mode. Dokl. Natl. Acad. Sci. Belarus 2021, 65, 111–118. (In Russian) [Google Scholar] [CrossRef]
- Bolisetty, S. Novel Process and Manufactur of Multi crystalline Solar Cell. 2009. Available online: http://liu.diva-portal.org/smash/get/diva2:210630/FULLTEXT01.pdf (accessed on 19 December 2023).
- Peter, A.P.; Meersschaut, J.; Richard, O.; Moussa, A.; Steenbergen, J.; Schaekers, M.; Adelmann, C. Phase formation and morphology of nickel silicide thin films synthesized by catalyzed chemical vapor reaction of nickel with silane. Chem. Mater. 2015, 27, 245–254. [Google Scholar] [CrossRef]
- Guo, X.; Yu, H.; Jiang, Y.L.; Ru, G.P.; Zhang, D.W.; Li, B.Z. Study of nickel silicide formation on Si (1 1 0) substrate. Appl. Surf. Sci. 2011, 257, 10571–10575. [Google Scholar] [CrossRef]
- Azimirad, R.; Kargarian, M.; Akhavan, O.; Moshfegh, A.Z. Improved thermal stability of NiSi nanolayer in Ni-Si Co-sputtered structure. Int. J. Nanosci. Nanotechnol. 2011, 7, 14–20. [Google Scholar]
- Bhaskaran, M.; Sriram, S.; Holland, A.S.; Evans, P.J. Characterisation of nickel silicide thin films by spectroscopy and microscopy techniques. Micron 2009, 40, 99–103. [Google Scholar] [CrossRef]
- Tinani, M.; Mueller, A.; Gao, Y.; Irene, E.A.; Hu, Y.Z.; Tay, S.P. In situ real-time studies of nickel silicide phase formation. J. Vac. Sci. Technol. B 2001, 19, 376–383. [Google Scholar] [CrossRef]
- Karabko, A.; Dragašius, E. NiSi and Ni (Pd) Si as possible interconnect and electrode materials for film bulk acoustic resonators and microelectromechanical systems. J. Vibroeng. 2013, 15, 196–203. [Google Scholar]
- Geetha Priyadarshini, B.; Aich, S.; Chakraborty, M. On the microstructure and interfacial properties of sputtered nickel thin film on Si (1 0 0). Bull. Mater. Sci. 2014, 37, 1265–1273. [Google Scholar] [CrossRef]
- Hurtado, C.; Ciampi, S. Oxidative Damage during the Operation of Si(211)-Based Triboelectric Nanogenerators. Surfaces 2023, 6, 281–290. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Lapitskaya, V.A.; Chizhik, S.A.; Warcholinski, B.; Gilewicz, A. Effect of Atmosphere During Deposition on the Morphology, Mechanical Properties and Microfriction of Zr-Based Coatings. Adv. Struct. Mater. 2022, 155, 271–319. [Google Scholar]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapitskaya, V.; Trukhan, R.; Kuznetsova, T.; Solovjov, J.; Chizhik, S.; Pilipenko, V.; Liutsko, K.; Nasevich, A.; Douhal, M. Microstructure and Properties of Thin-Film Submicrostructures Obtained by Rapid Thermal Treatment of Nickel Films on Silicon. Surfaces 2024, 7, 196-207. https://doi.org/10.3390/surfaces7020013
Lapitskaya V, Trukhan R, Kuznetsova T, Solovjov J, Chizhik S, Pilipenko V, Liutsko K, Nasevich A, Douhal M. Microstructure and Properties of Thin-Film Submicrostructures Obtained by Rapid Thermal Treatment of Nickel Films on Silicon. Surfaces. 2024; 7(2):196-207. https://doi.org/10.3390/surfaces7020013
Chicago/Turabian StyleLapitskaya, Vasilina, Ruslan Trukhan, Tatyana Kuznetsova, Jaroslav Solovjov, Sergei Chizhik, Vladimir Pilipenko, Karyna Liutsko, Anastasiya Nasevich, and Maksim Douhal. 2024. "Microstructure and Properties of Thin-Film Submicrostructures Obtained by Rapid Thermal Treatment of Nickel Films on Silicon" Surfaces 7, no. 2: 196-207. https://doi.org/10.3390/surfaces7020013
APA StyleLapitskaya, V., Trukhan, R., Kuznetsova, T., Solovjov, J., Chizhik, S., Pilipenko, V., Liutsko, K., Nasevich, A., & Douhal, M. (2024). Microstructure and Properties of Thin-Film Submicrostructures Obtained by Rapid Thermal Treatment of Nickel Films on Silicon. Surfaces, 7(2), 196-207. https://doi.org/10.3390/surfaces7020013