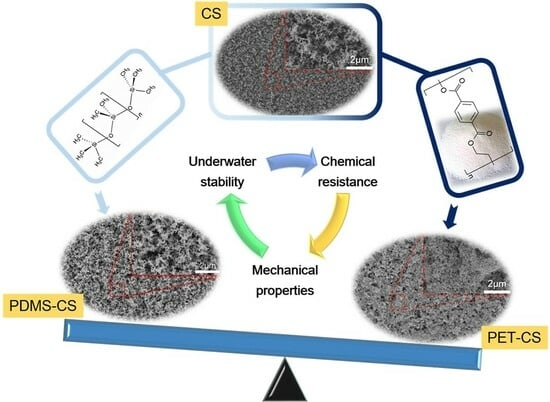
A Route towards Durable Underwater Stable Superhydrophobic Surfaces: PET-Reinforced Candle Soot Layers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of CS Surface
2.2.2. Preparation of PDMS-CS Composite Coatings
2.2.3. Preparation of PET-CS Composite Coatings
2.3. Characterization
3. Results and Discussion
3.1. Surface Morphologies and Chemical Compositions
3.2. Surface Wettability of the Coatings
3.3. Underwater Stability
3.4. Sandpaper Scratching Test and Analysis
3.5. Water Impact Tests
3.6. Chemical Durability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marmur, A. Underwater superhydrophobicity: Theoretical feasibility. Langmuir 2006, 22, 1400–1402. [Google Scholar] [CrossRef]
- Yu, N.; Li, Z.R.; McClelland, A.; Kim, C.J.C. Combined theory and experimental verification of plastron stability on superhydrophobic surface. In Proceedings of the 2022 IEEE 35th International Conference on Micro Electro Mechanical Systems Conference (MEMS), Tokyo, Japan, 9–13 January 2022; IEEE: Piscataway Township, NJ, USA, 2022; pp. 99–101. [Google Scholar] [CrossRef]
- Smyrnakis, A.; Ioannou, D.; Ellinas, K.; Tserepi, A.; Gogolides, E. Real-Time Monitoring and Quantification of Underwater Superhydrophobicity. Adv. Mater. Interfaces 2022, 9, 2101393. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, S.; Lv, P.; Xue, Y.; Su, Q.; Duan, H. Ultimate stable underwater superhydrophobic state. Phys. Rev. Lett. 2017, 119, 134501. [Google Scholar] [CrossRef]
- Breveleri, J.; Mohammadshahi, S.; Dunigan, T.; Ling, H. Plastron restoration for underwater superhydrophobic surface by porous material and gas injection. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132319. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, S.; Huang, T.Y.; Dong, A.; Cao, D.; Li, H.; Duan, H. Superrepellency of underwater hierarchical structures on Salvinia leaf. Proc. Natl. Acad. Sci. USA 2020, 117, 2282–2287. [Google Scholar] [CrossRef]
- Choi, W.; Kang, M.; Park, J.Y.; Jeong, H.E.; Lee, S.J. Enhanced air stability of superhydrophobic surfaces with flexible overhangs of re-entrant structures. Phys. Fluids 2021, 33, 022001. [Google Scholar] [CrossRef]
- Deepak, J.; Arindam, D. Longevity of plastron layer in underwater vapor stable superhydrophobic surfaces under different atmospheric condition and drag measurements. Bull. Am. Phys. Soc. 2022, 67, 19. [Google Scholar]
- Dai, Z.; Ding, S.; Lei, M.; Li, S.; Xu, Y.; Zhou, Y.; Zhou, B. A superhydrophobic and anti-corrosion strain sensor for robust underwater applications. J. Mater. Chem. A 2021, 9, 15282–15293. [Google Scholar] [CrossRef]
- Ni, Y.; Huang, J.; Li, S.; Dong, X.; Zhu, T.; Cai, W.; Lai, Y. Robust superhydrophobic rGO/PPy/PDMS coatings on a polyurethane sponge for underwater pressure and temperature sensing. ACS Appl. Mater. Interfaces 2021, 13, 53271–53281. [Google Scholar] [CrossRef]
- Nazhipkyzy, M.; Nurgain, A.; Florent, M.; Policicchio, A.; Bandosz, T.J. Magnetic soot: Surface properties and application to remove oil contamination from water. J. Environ. Chem. Eng. 2019, 7, 103074. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Ganeva, R.R.; Stamenov, G.S.; Chaushev, T.A. Superhydrophobic soot coated quartz crystal microbalances: A novel platform for human spermatozoa quality assessment. Sensors 2019, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Tao, Y.; Chen, J.; Dai, J.; Song, T.; Ruan, M.; Wang, Z.L. Carbon nanoparticles on carbon fabric for flexible and high-performance field emitters. Adv. Funct. Mater. 2011, 21, 2150–2154. [Google Scholar] [CrossRef]
- Yuan, L.; Dai, J.; Fan, X.; Song, T.; Tao, Y.T.; Wang, K.; Wang, Z.L. Self-cleaning flexible infrared nanosensor based on carbon nanoparticles. ACS Nano 2011, 5, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, R.; Majhy, B.; Sen, A.K. Facile fabrication and characterization of a PDMS-derived candle soot coated stable biocompatible superhydrophobic and superhemophobic surface. ACS Appl. Mater. Interfaces 2017, 9, 31170–31180. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, L.; Ma, F.; Liu, L. Candle soot coated nickel foam for facile water and oil mixture separation. RSC Adv. 2014, 4, 7132–7135. [Google Scholar] [CrossRef]
- Do, V.T.; Tran, N.G.; Chun, D.M. Fabrication of robust superhydrophobic micro-nano hierarchical surface structure using compression molding with carbon soot nanoparticles and thermoplastic polymer. Polymer 2022, 251, 124893. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Bressler, A.H.; Abolghasemibizaki, M.; Mohammadi, R. Rapid synthesis of inherently robust and stable superhydrophobic carbon soot coatings. Appl. Surf. Sci. 2016, 369, 341–347. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Ben, K.; Chen, Z.; Wang, Y.; Guan, Z. Transparent, durable and thermally stable PDMS-derived superhydrophobic surfaces. Appl. Surf. Sci. 2015, 339, 94–101. [Google Scholar] [CrossRef]
- Celik, N.; Kiremitler, N.B.; Ruzi, M.; Onses, M.S. Waxing the soot: Practical fabrication of all-organic superhydrophobic coatings from candle soot and carnauba wax. Prog. Org. Coat. 2021, 153, 106169. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, M.; Zhang, J.; Tan, G.; Pan, Y.; Lai, Y.; Chen, Z. An underwater stable superhydrophobic surface for robust ultra-long-lasting biofouling resistance. Chem. Eng. J. 2023, 462, 142091. [Google Scholar] [CrossRef]
- Chen, Y.; Su, N.; Zhang, K.; Zhu, S.; Zhao, L.; Fang, F.; Ren, L.; Guo, Y. In-depth analysis of the structure and properties of two varieties of natural luffa sponge fibers. Materials 2017, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.M.; Gao, L.; Shields, C.W.; Smith, M.; Efimenko, K.; Cushing, K.; Genzer, J.; López, G.P. Elastomeric microparticles for acoustic mediated bioseparations. J. Nanobiotechnol. 2013, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- El-Saftawy, A.A.; Elfalaky, A.; Ragheb, M.; Zakhary, S. Electron beam induced surface modifications of PET film. Radiat. Phys. Chem. 2014, 102, 96–102. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Mohammadi, R. Interactions of superhydrophobic carbon soot coatings with short alkyl chain alcohols and fluorocarbon solutions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 715–724. [Google Scholar] [CrossRef]
- Yang, L.; Fu, H.; Yang, C.; Tian, W.; Wu, P.; Jiang, W. Carbon soot with arbitrary wettability deposited on solid surface by ethanol flame method. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123576. [Google Scholar] [CrossRef]
- Sutar, R.S.; Latthe, S.S.; Sargar, A.M.; Patil, C.E.; Jadhav, V.S.; Patil, A.N.; Xing, R. Spray deposition of PDMS/candle soot nps composite for self-cleaning superhydrophobic coating. Macromol. Symp. 2020, 393, 2000031. [Google Scholar] [CrossRef]
- Lin, X.; Park, S.; Choi, D.; Heo, J.; Hong, J. Mechanically durable superhydrophobic PDMS-candle soot composite coatings with high biocompatibility. J. Ind. Eng. Chem. 2019, 74, 79–85. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, R.; Fu, H.; Xu, J.; Jing, Y.; Xu, G.; Hou, X. Efficient oil–water separation coating with robust superhydrophobicity and high transparency. Sci. Rep. 2022, 12, 2187. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Zhou, H.; Tian, G. Preparation and evaluation of PDMS/carbon soot particles superhydrophobic biomimetic composite coating with self-cleaning and durability. Biomimetics 2022, 7, 132. [Google Scholar] [CrossRef]
- Xue, C.H.; Zhang, P.; Ma, J.Z.; Ji, P.T.; Li, Y.R.; Jia, S.T. Long-lived superhydrophobic colorful surfaces. Chem. Commun. 2013, 49, 3588–3590. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Han, Z.; Wang, Y.; Pan, Y.; Jie, X. A Route towards Durable Underwater Stable Superhydrophobic Surfaces: PET-Reinforced Candle Soot Layers. Surfaces 2024, 7, 225-237. https://doi.org/10.3390/surfaces7020015
Wu X, Han Z, Wang Y, Pan Y, Jie X. A Route towards Durable Underwater Stable Superhydrophobic Surfaces: PET-Reinforced Candle Soot Layers. Surfaces. 2024; 7(2):225-237. https://doi.org/10.3390/surfaces7020015
Chicago/Turabian StyleWu, Xinghua, Zhaokang Han, Yuchao Wang, Yutong Pan, and Xiaohua Jie. 2024. "A Route towards Durable Underwater Stable Superhydrophobic Surfaces: PET-Reinforced Candle Soot Layers" Surfaces 7, no. 2: 225-237. https://doi.org/10.3390/surfaces7020015
APA StyleWu, X., Han, Z., Wang, Y., Pan, Y., & Jie, X. (2024). A Route towards Durable Underwater Stable Superhydrophobic Surfaces: PET-Reinforced Candle Soot Layers. Surfaces, 7(2), 225-237. https://doi.org/10.3390/surfaces7020015