Harbour Seals: Population Structure, Status, and Threats in a Rapidly Changing Environment
Abstract
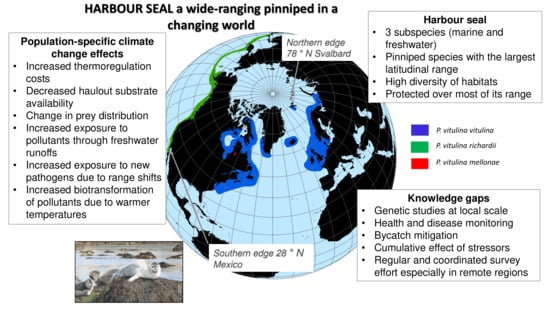
:1. Introduction
2. Distribution and Genetic Structure
3. Status of the Main Populations and Current Threats
3.1. Western Pacific Coast
3.2. Eastern Pacific Coast
3.2.1. Alaska
3.2.2. British Columbia, Washington, Oregon, and California
3.3. Western North Atlantic Coast
3.4. Greenland
3.5. Svalbard
3.6. Iceland and Faroe Islands
3.7. Continental Europe
3.7.1. Northern Europe
3.7.2. Southern Europe
3.8. United Kingdom and Ireland
4. Environmental Changes and Potential Consequences on Harbour Seals Populations
4.1. Increased Temperatures and Extreme Weather Events Affect Haul-Out Patterns
4.2. Changes in Physical Habitat Affect Distribution Patterns
4.3. Large-Scale Oceanic Events and Changes in Community Structure Affect Foraging
4.4. Shift in Pathogen Ranges May Affect Populations’ Trajectories
4.5. Increased Anthropogenic Disturbances May Affect Pristine Populations
5. Management and Knowledge Gaps under Rapidly Increasing Environmental Changes
5.1. Current Management Framework
- Under the Marine Mammal Protection Act in the USA (enacted in 1972) throughout its range, although subsistence hunting and traditional use are permitted for coastal Alaskan natives.
- By the Marine Mammal Regulations under the Fisheries Act in the non-Arctic part of Canada (since 1967 for the Pacific population and 1970 for the Atlantic population), while in the Arctic, subsistence hunting is permitted for both the marine and fresh water subspecies. The species is not managed in Nunavut.
- In Greenland (since 2010).
- In Iceland (since 2019).
- In Svalbard (since 1970s).
- Under the EU Habitats and Species Directive 1992 (Council Directive 92/43/EEC on the Conservation of natural habitats and of wild fauna and flora). The species is listed in Annex II (species requiring the designation of special areas of conservation, SAC, or marine protected areas, MPAs) and V (species whose taking from the wild can be restricted by European law). The monitoring of their population abundance and distribution is requested under the Marine Strategy Framework Directive (MSFD).
- In the Russian Federation (since 1975).
- In Japan under the Wildlife Protection, Management and the Hunting Law (since 2003) with some specific local population control plans.
5.2. Knowledge Gaps
5.3. Adaptive Management of Harbour Seals Following a Precautionary Approach
6. Conclusions
Funding
Conflicts of Interest
References
- Shaughnessy, P.D.; Fay, F.H. A review of the taxonomy and nomenclature of North Pacific Harbour seals. J. Zool. 1977, 182, 385–419. [Google Scholar] [CrossRef]
- Teilmann, J.; Galatius, A. Harbor Seal. In Encyclopedia of Marine Mammals, 3rd ed.; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 451–455. [Google Scholar]
- Rosing-Asvid, A.; Teilmann, J.; Olsen, M.T.; Dietz, R. Deep diving harbor seals (Phoca vitulina) in South Greenland: Movements, diving, haul-out and breeding activities described by telemetry. Polar Biol. 2020, 43, 359–368. [Google Scholar] [CrossRef]
- Merkel, B.; Lydersen, C.; Yoccoz, N.G.; Kovacs, K.M. The World’s Northernmost Harbour Seal Population—How Many Are There? PLoS ONE 2013, 8, e67576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávez-rosales, S.; Gardner, S.C. Recent harbour seal (Phoca vitulina richardsi) pup sightings in Magdalena Bay, Baja California Sur, Mexico. Aquat. Mammals 1999, 25, 169–171. [Google Scholar]
- Boveng, P.L.; Bengtson, J.L.; Withrow, D.E.; Cesarone, J.C.; Simpkins, M.A.; Frost, K.J.; Burns, J.J. The abundance of harbor seals in the Gulf of Alaska. Mar. Mammal Sci. 2003, 19, 111–127. [Google Scholar] [CrossRef]
- Vincent, C.; McConnell, B.J.; Delayat, S.; Elder, J.-F.; Gautier, G.; Ridoux, V. Winter habitat use of harbour seals (Phoca vitulina) fitted with FastlocTMGPS/GSM tags in two tidal bays in France. NAMMCO Sci. Publ. 2010, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- London, J.M.; Hoef, J.M.; Jeffries, S.J.; Lance, M.M.; Boveng, P.L. Haul-out behavior of harbor seals (Phoca vitulina) in Hood Canal, Washington. PLoS ONE 2012, 7, e38180. [Google Scholar] [CrossRef] [Green Version]
- Dietz, R.; Teilmann, J.; Andersen, S.M.; Rige, F.; Olsen, M.T. Movements and site fidelity of harbour seals (Phoca vitulina) in Kattegat, Denmark, with implications for the epidemiology of the phocine distemper virus. ICES J. Mar. Sci. 2013, 70, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Blanchet, M.-A.; Lydersen, C.; Ims, R.A.; Lowther, A.D.; Kovacs, K.M. Harbour seal Phoca vitulina movement patterns in the high-arctic archipelago of Svalbard, Norway. Aquat. Biol. 2014, 21. [Google Scholar] [CrossRef] [Green Version]
- Womble, J.N.; Gende, S.M. Post-Breeding Season Migrations of a Top Predator, the Harbor Seal (Phoca vitulina richardii), from a Marine Protected Area in Alaska. PLoS ONE 2013, 8, e55386. [Google Scholar] [CrossRef] [Green Version]
- Reijnders, P.J.H.; Brasseur, S.M.J.M.; Meesters, E.H.W.G. Earlier pupping in harbour seals, Phoca vitulina. Biol. Lett. 2010, 6, 854–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, K.J.; Hall, A.J.; Scholl, G.; Debier, C.; Thomé, J.P.; Eppe, G.; Adam, C.; Bennett, K.A. Investigating decadal changes in persistent organic pollutants in Scottish grey seal pups. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Brasseur, S.M.; Reijnders, P.J.; Cremer, J.; Meesters, E.; Kirkwood, R.; Jensen, L.F.; Jeβ, A.; Galatius, A.; Teilmann, J.; Aarts, G. Echoes from the past: Regional variations in recovery within a harbour seal population. PLoS ONE 2018, 13, e0189674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, K.M.; Lydersen, C. Climate change impacts on seals and whales in the North Atlantic Arctic and adjacent shelf seas. Sci. Prog. 2008, 91, 117–150. [Google Scholar] [CrossRef]
- Forcada, J.; Trathan, P.N.; Reid, K.; Murphy, E.J. The effects of global climate variability in pup production of antarctic fur seals. Ecology 2005, 86, 2408–2417. [Google Scholar] [CrossRef]
- Descamps, S.; Aars, J.; Fuglei, E.; Kovacs, K.M.; Lydersen, C.; Pavlova, O.; Pedersen, Å.Ø.; Ravolainen, V.; Strøm, H. Climate change impacts on wildlife in a High Arctic archipelago—Svalbard, Norway. Glob. Chang. Biol. 2017, 23, 490–502. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Masson-Delmotte, V.; Zhai, P.; Tignor, M.; Poloczanska, E.; Mintenbeck, K.; Alegría, A.; Nicolai, M.; Okem, A.; et al. Summary for Policymakers. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Available online: https://www.ipcc.ch/srocc/chapter/summary-for-policymakers/ (accessed on 30 December 2020).
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T.; et al. Global imprint of climate change on marine life. Nat. Clim. Chang. 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Kovacs, K.M.; Aguilar, A.; Aurioles, D.; Burkanov, V.; Campagna, C.; Gales, N.; Gelatt, T.; Goldsworthy, S.D.; Goodman, S.J.; Hofmeyr, G.J.; et al. Global threats to pinnipeds. Mar. Mammal Sci. 2012, 28, 414–436. [Google Scholar] [CrossRef]
- Dippner, J.W.; Vuorinen, I.; Daunys, D.; Flinkman, J.; Halkka, A.; Köster, F.W.; Lehikoinen, E.; MacKenzie, B.R.; Möllmann, C.; Møhlenberg, F.; et al. Climate-related Marine Ecosystem Change. In Assessment of Climate Change for the Baltic Sea Basin; Springer: Berlin/Heidelberg, Germany, 2008; pp. 309–377. [Google Scholar]
- Hamilton, C.D.; Kovacs, K.M.; Ims, R.A.; Aars, J.; Lydersen, C. An Arctic predator–prey system in flux: Climate change impacts on coastal space use by polar bears and ringed seals. J. Anim. Ecol. 1054, 2017, 86–1064. [Google Scholar] [CrossRef]
- Hindell, M.A.; Sumner, M.; Bestley, S.; Wotherspoon, S.; Harcourt, R.G.; Lea, M.A.; Alderman, R.; McMahon, C.R. Decadal changes in habitat characteristics influence population trajectories of southern elephant seals. Glob. Chang. Biol. 2017, 23, 5136–5150. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.P.; Huckstadt, L.A.; Crocker, D.E.; McDonald, B.I.; Goebel, M.E.; Fedak, M.A. Approaches to Studying Climatic Change and its Role on the Habitat Selection of Antarctic Pinnipeds. Integr. Comp. Biol. 2010, 1018, 50–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, C.; Hobday, A.J.; Chambers, L.E. Using expert elicitation to rank ecological indicators for detecting climate impacts on Australian seabirds and pinnipeds. Ecol. Indic. 2018, 95, 637–644. [Google Scholar] [CrossRef]
- Rugh, D.; DeMaster, D.; Rooney, A.; Breiwick, J.; Shelden, K.; Moore, S. A review of bow-head whale (Balaena mysticetus) stock identity. J. Cetacean Res. Manag. 2003, 7, 1–12. [Google Scholar]
- Berta, A.; Churchill, M. Pinniped taxonomy: Review of currently recognized species and subspecies, and evidence used for their description. Mammals Rev. 2012, 42, 207–234. [Google Scholar] [CrossRef]
- Westlake, R.L.; O’Corry-Crowe, G.M. Macrogeographic Structure and Patterns of Genetic Diversity in Harbor Seals (Phoca vitulina) from Alaska to Japan. J. Mammal. 2002, 1111, 83–1126. [Google Scholar] [CrossRef] [Green Version]
- DFO. Recovery Strategy for the Harbour Seal, Lacs Des Loups Marins Subspecies (Phoca vitulina mellonae). 2018. Available online: https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/recovery-strategies/harbour-seal-lacs-loups-marins.html (accessed on 6 May 2019).
- Stanley, H.F.; Casey, S.; Carnahan, J.M.; Goodman, S.; Harwood, J.; Wayne, R.K. Worldwide patterns of mitochondrial DNA differentiation in the harbor seal (Phoca vitulina). Mol. Biol. Evol. 1996, 13, 368–382. [Google Scholar] [CrossRef] [Green Version]
- Olsen, M.T.; Andersen, L.W.; Dietz, R.; Teilmann, J. Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Mol. Ecol. 2014, 815–831. [Google Scholar] [CrossRef]
- Andersen, L.; Olsen, M.T. Distribution and population structure of North Atlantic harbour seals (Phoca vitulina). NAMMCO Sci. Publ. 2010, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Herreman, J.K.; Blundell, G.M.; Mcdonald, D.B.; Ben-David, M. Asymmetrical male-mediated gene flow between Harbor seal (Phoca vitulina) populations in Alaska. Can. J. Zool. 2009, 87, 498–507. [Google Scholar] [CrossRef]
- Goodman, S.J. Patterns of extensive genetic differentiation and variation among European harbor seals (Phoca vitulina vitulina) revealed using microsatellite DNA poly morphism. Mol. Biol. Evol. 1998, 15, 104–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Corry-Crowe, G.M.; Martien, K.K.; Taylor, B.L. The Analysis of Population Genetic Structure in Alaskan Harbor Seals, Phoca vitulina, as a Framework for the Identification of Management Stocks; Southwest Fisheries Science Center Administrative Report LJ-03-08. Available online: https://repository.library.noaa.gov/view/noaa/19225 (accessed on 16 December 2004).
- Mizuno, M.; Kobayashi, M.; Sasaki, T.; Haneda, T.; Masubuchi, T. Current population genetics of Japanese harbor seals: Two distinct populations found within a small area. Mar. Mammal Sci. 2020, 36, 915–924. [Google Scholar] [CrossRef]
- Steingass, S.; Horning, M.; Bishop, A.M. Space use of Pacific harbor seals (Phoca vitulina richardii) from two haul-out locations along the Oregon coast. PLoS ONE 2019, 14, e0219484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, M.; Sasaki, T.; Kobayashi, M.; Haneda, T.; Masubuchi, T. Mitochondrial DNA reveals secondary contact in Japanese harbour seals, the southernmost population in the western Pacific. PLoS ONE 2018, 13, e0191329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muto, M.M.; Helker, V.T.; Delean, B.J.; Angliss, R.P.; Boveng, P.L.; Breiwick, J.M.; Brost, B.M.; Cameron, M.F.; Clapham, P.J.; Dahle, S.P.; et al. Alaska Marine Mammal Stock Assessments. 2019. Available online: https://repository.library.noaa.gov/view/noaa/25642 (accessed on 31 July 2020).
- Andersen, L.W.; Lydersen, C.; Frie, A.K.; Rosing-Asvid, A.; Hauksson, E.; Kovacs, K.M. A population on the edge: Genetic diversity and population structure of the world’s northernmost harbour seals (Phoca vitulina). Biol. J. Linn. Soc. 2011, 102, 420–439. [Google Scholar] [CrossRef] [Green Version]
- SCOS. Scientific Advice on Matters Related to the Management of Seal Populations. 2018. Available online: http://www.smru.st-andrews.ac.uk/files/2019/05/SCOS-2018.pdf (accessed on 31 May 2019).
- Olsen, M.T.; Islas, V.; Graves, J.A.; Onoufriou, A.; Vincent, C.; Brasseur, S.; Frie, A.K.; Hall, A.J. Genetic population structure of harbour seals in the United Kingdom and neighbouring waters. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Bjørge, A.; Desportes, G.; Waring, G.; Rosing-Asvid, A. Introduction: The harbour seal (Phoca vitulina)—A global perspective. In NAMMCO Scientific Publications 8; UiT The Arctic University of Norway: Tromsø, Norway, 2010; pp. 7–14. [Google Scholar]
- Lowry, L.; Phoca vitulina. In the IUCN Red List of Threatened Species 2016. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T17013A45229114.en (accessed on 6 May 2019).
- Kobayashi, Y.; Kariya, T.; Chishima, J.; Fujii, K.; Wada, K.; Baba, S.; Itoo, T.; Nakaoka, T.; Kawashima, M.; Saito, S.; et al. Population trends of the Kuril harbour seal Phoca vitulina stejnegeri from 1974 to 2010 in southeastern Hokkaido, Japan. Endanger. Species Res. 2014, 24, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Rosing-Asvid, A. Catch history and status of the harbour seal (Phoca vitulina) in Greenland. NAMMCO Sci. Publ. 2010, 8. [Google Scholar] [CrossRef] [Green Version]
- Niizuma, A.; Hayama, S. A.; Hayama, S. A review of the taxonomy of the Kuril seal and other members of the genus Phoca (sensu stricto). In Ecology and Protection of Kuril Seal; Wada, K., Itoo, T., Niizum, A., Hayama, S., Suzuki, M., Eds.; Tokai University Press: Tokyo, Japan, 1986; pp. 1–18. [Google Scholar]
- Trukhin, A. Current Status of Pinnnipeds in the Sea of Okhotsk. In Proceedings of the Fourth Workshop on the Okhotsk Sea and Adjacent Areas, Abashiri, Japan, 27–29 August 2008. [Google Scholar]
- Mamaev, E.G. A new method of counting Phoca vitulina ssp. Stejnegeri (Phocida, Carnivora) on the Commander Islands (Russia). Nat. Conserv. Res. 2018, 3. [Google Scholar] [CrossRef]
- Reijnders, P.J.H.; Brasseur, S.; Toorn, J.V.D.; Boyd, I.; Harwood, J.; Lowry, L. Seals, Fur Seals, Sea Lions and Walruses: Status of Pinnipeds and Conservation Action Plan; International Union for the Conservation of Nature and Natural Resources (IUCN): Gland, Switzerland, 1993. [Google Scholar]
- Allen, B.; Angliss, R. Alaska Marine Mammal Stock Assessments. 2012. Available online: https://www.fisheries.noaa.gov/resource/document/alaska-marine-mammal-stock-assessments-2012 (accessed on 6 May 2019).
- Jansen, J.K.; Boveng, P.L.; Hoef, J.M.V.; Dahle, S.P.; Bengtson, J.L. Natural and human effects on harbor seal abundance and spatial distribution in an Alaskan glacial fjord. Mar. Mammal Sci. 2015, 31, 66–89. [Google Scholar] [CrossRef]
- Lydersen, C.; Assmy, P.; Falk-Petersen, S.; Kohler, J.; Kovacs, K.M.; Reigstad, M.; Steen, H.; Strøm, H.; Sundfjord, A.; Varpe, Ø.; et al. The importance of tidewater glaciers for marine mammals and seabirds in Svalbard, Norway. J. Mar. Syst. 2014, 129, 452–471. [Google Scholar] [CrossRef]
- Boveng, P.L.; Hoef, J.M.V.; Withrow, D.E.; London, J.M. A Bayesian Analysis of Abundance, Trend, and Population Viability for Harbor Seals in Iliamna Lake, Alaska. Risk Anal. 1988, 2018, 38–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, J.M.; Van Lanen, J.M.; Holen, D.L.; Zimpelman, G.; Jones, B.E.; Withrow, D.E.; Askoak, T.; Aderman, H.; O’Corey-Crowe, G. Integrating Local Traditional Knowledge and Subsistence Use Patterns with Aerial Surveys to Improve Scientific and Local Understanding of Iliamna Lake Seals. Alaska Department of Fish and Game, Division of Subsistence, 2016. Technical Paper No. 416. Anchorage. 2016. Available online: https://www.fisheries.noaa.gov/resource/peer-reviewed-research/integrating-local-traditional-knowledge-and-subsistence-use (accessed on 1 June 2016).
- Hauser, D.D.W.; Allen, C.S.; Rich, H.B.; Quinn, T.P. Resident harbor seals (Phoca vitulina) in Iliamna Lake, Alaska: Summer diet and partial consumption of adult sockeye salmon (Oncorhynchus nerka). Aquat. Mammals 2008, 34, 303–309. [Google Scholar] [CrossRef]
- Small, R.J.; Pendleton, G.W.; Pitcher, K.W. Trends in abundance of Alaska harbor seals, 1983–2001. Mar. Mammal Sci. 2003, 19, 44–362. [Google Scholar] [CrossRef]
- Adkison, M.D.; Quinn, T.J.; Small, R.J. Evaluation of the Alaska harbor seal (Phoca vitulina) population survey: A simulation study. Mar. Mammal Sci. 2003, 19, 764–790. [Google Scholar] [CrossRef]
- Small, R.J.; Boveng, P.L.; Byrd, G.V.; Withrow, D.E. Harbor seal population decline in the Aleutian Archipelago. Mar. Mammal Sci. 2008, 24, 845–863. [Google Scholar] [CrossRef] [Green Version]
- Jemison, L.A.; Pendleton, G.W.; Wilson, C.A.; Small, R.J. Long-term trends in harbor seal numbers at Tugidak Island and Nanvak Bay, Alaska. Mar. Mammal Sci. 2006, 22, 339–360. [Google Scholar] [CrossRef]
- Pitcher, K.W. Major Decline in Number of Harbor Seals, Phoca vitulina richardsi, on Tugidak Island, Gulf of Alaska. Mar. Mammal Sci. 1990, 6, 121–134. [Google Scholar] [CrossRef]
- Frost, K.J.; Lowry, L.F.; Hoef, J.M.V. Monitoring the trend of harbor seals in Prince William Sound, Alaska, after the Exxon Valdez oil spill. Mar. Mammal Sci. 1999, 15, 494–506. [Google Scholar] [CrossRef]
- Wang, D.; Atkinson, S.; Hoover-Miller, A.; Shelver, W.L.; Li, Q.X. Organic halogenated contaminants in mother–fetus pairs of harbor seals (Phoca vitulina richardii) from Alaska, 2000–2002. J. Hazard. Mater. 2012, 224, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Hoover-Miller, A.; Armato, P. Harbor seal use of glacier ice and terrestrial haul-outs in the Kenai Fjords, Alaska. Mar. Mammal Sci. 2018, 34, 616–644. [Google Scholar] [CrossRef] [Green Version]
- Mathews, E.A.; Pendleton, G.W. Declines in harbor seal (Phoca vitulina) numbers in Glacier Bay National Park, Alaska, 1992-2002. Mar. Mammal Sci. 2006, 22, 167–189. [Google Scholar] [CrossRef]
- Womble, J.N.; Pendleton, G.W.; Mathews, E.A.; Blundell, G.M.; Bool, N.M.; Gende, S.M. Harbor seal (Phoca vitulina richardii) decline continues in the rapidly changing landscape of Glacier Bay National Park, Alaska 1992–2008. Mar. Mammal Sci. 2010, 26, 686–697. [Google Scholar] [CrossRef]
- Womble, J.N.; Hoef, J.M.V.; Gende, S.M.; Mathews, E.A. Calibrating and adjusting counts of harbor seals in a tidewater glacier fjord to estimate abundance and trends 1992 to 2017. Ecosphere 2020, 11, e03111. [Google Scholar] [CrossRef]
- Miller, A.J.; Cayan, D.R.; Barnett, T.P.; Graham, N.E.; Oberhuber, J.M. The 1976-77 Climate Shift of the Pacific Ocean. Oceanography 1994, 7, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Trenberth, K.E.; Hurrell, J.W. Decadal atmosphere-ocean variations in the Pacific. Clim. Dyn. 1994, 9, 303–319. [Google Scholar] [CrossRef]
- Trites, A.W.; Miller, A.J.; Maschner, H.D.; Alexander, M.A.; Bograd, S.J.; Calder, J.A.; Capotondi, A.; Coyle, K.O.; Lorenzo, E.D.; Finney, B.P.; et al. Bottom-up forcing and the decline of Steller sea lions (Eumetopias jubatus) in Alaska: Assessing the ocean climate hypothesis. Fish. Oceanogr. 2006, 16, 46–67. [Google Scholar] [CrossRef] [Green Version]
- Bond, N.A.; Cronin, M.F.; Freeland, H.; Mantua, N. Causes and impacts of the 2014 warm anomaly in the NE Pacific. Geophys. Res. Lett. 2015, 42, 3414–3420. [Google Scholar] [CrossRef]
- Batten, S.D.; Raitsos, D.E.; Danielson, S.; Hopcroft, R.; Coyle, K.; McQuatters-Gollop, A. Interannual variability in lower trophic levels on the Alaskan Shelf. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 147, 58–68. [Google Scholar] [CrossRef]
- Olesiuk, P.F. An Assessment of Population Trends and Abundance of Harbour Seals (Phoca vitulina) in British Columbia. DFO Can. Sci. Advis. Sec. Res. Doc 2009, 105. [Google Scholar]
- Harbour seal (Phoca vitulina) Counts and Haulout Locations in the Strait of Georgia, British Columbia Coast. Available online: https://open.canada.ca/data/en/dataset/be5a4ba8-79dd-4787-bf8a-0d460d25954c (accessed on 6 May 2019).
- Huber, H.R.; Jeffries, S.J.; Brown, R.F.; Delong, R.L.; Vanblaricom, G. Correcting aerial survey counts of harbor seals (Phoca vitulina richardsi) in Washington and Oregon. Mar. Mammal Sci. 2001, 17, 276–293. [Google Scholar] [CrossRef]
- Brown, R.F.; Wright, B.E.; Riemer, S.D.; Laake, J. Trends in abundance and current status of harbor seals in Oregon: 1977-2003. Mar. Mammal Sci. 2005, 21, 657–670. [Google Scholar] [CrossRef]
- Sydeman, W.J.; Allen, S.G. Pinniped population dynamics in central California: Correlations with sea surface temperature and upwelling indices. Mar. Mammal Sci. 1999, 15, 446–461. [Google Scholar] [CrossRef]
- Brown, R.F.; Jeffries, S.J.; Wright, B.E. Conductivity-Temperature-Depth Profiling of the Columbia River Mouth Using Pacific Harbor Seals as Sampling Platforms. 2013. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/a598459.pdf (accessed on 6 May 2019).
- Carretta, J.V.; Oleson, E.M.; Weller, D.W.; Lang, A.R.; Forney, K.A.; Baker, J.D.; Hanson, B.; Martien, K.K.; Muto, M.; Orr, A.J.; et al. U.S. Pacific Marine Mammal Stock Assessments, 2013. Available online: https://repository.library.noaa.gov/view/noaa/4772 (accessed on 4 January 2021).
- Steingass, S.M. Habitat Use, Spatial Ecology, and Stable Isotope Variability of the Pacific Harbor Seal (Phoca vitulina richardii) along the Oregon Coast. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2018. [Google Scholar]
- Becker, B.H.; Press, D.T.; Allen, S.G. Modeling the effects of El Niño, density-dependence, and disturbance on harbor seal (Phoca vitulina) counts in Drakes Estero, California: 1997–2007. Mar. Mammal Sci. 2009, 25, 1–18. [Google Scholar] [CrossRef]
- Elorriaga-Verplancken, F.R.; Morales-Luna, L.; Heckel, G.; Schramm, Y. Foraging ecology of harbour seals (Phoca vitulina) and Northern elephant seals (Mirounga angustirostris) from Baja California, Mexico: Inferences from stable isotopes in pups. J. Mar. Biol. Assoc. U. K. 2016, 96, 903–908. [Google Scholar] [CrossRef]
- Waring, G.T.; Gilbert, J.R.; Belden, D.; Van Atten, A.; DiGiovanni, R.A., Jr. A review of the status of harbour seals (Phoca vitulina) in the Northeast United States of America. NAMMCO Sci. Publ. 2010, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Hammill, M.O.; Bowen, D.W.; Sjare, B. Status of harbour seals (Phoca vitulina) in Atlantic Canada. NAMMCO Sci. Publ. 2010, 8, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Boulva, J.; McLaren, I.A. Biology of the harbor seal, Phoca vitulina, in eastern Canada. Bull. Fish. Res. Can. 1979, 24. [Google Scholar] [CrossRef]
- NOAA. Harbor Seal (Phoca vitulina): Western North Atlantic Stock. Available online: https://archive.afsc.noaa.gov/nmml/PDF/sars/ao2006sehr-wn.pdf (accessed on 6 May 2019).
- Waring, G.T.; Digiovanni, R.A., Jr.; Josephson, E. 2012 Population Estimate for the Harbor Seal (Phoca vitulina concolor) in New England Waters. NOAA Tech. Memo. NMFS NE 2015, 235, 15. [Google Scholar] [CrossRef]
- Lucas, Z.; Stobo, W.T. Shark-inflicted mortality on a population of harbour seals (Phoca vitulina) at Sable Island, Nova Scotia. J. Zool. 2000, 252, 405–414. [Google Scholar] [CrossRef]
- Bowen, W.D.; Ellis, S.L.; Iverson, S.J.; Boness, D.J. Maternal and newborn life-history traits during periods of contrasting population trends: Implications for explaining the decline of harbour seals (Phoca vitulina), on Sable Island. J. Zool. 2003, 261, 155–163. [Google Scholar] [CrossRef]
- Johnston, D.W.; Frungillo, J.; Smith, A.; Moore, K.; Sharp, B.; Schuh, J.; Read, A.J. Trends in Stranding and bycatch Rates of Gray and Harbor Seals along the Northeastern Coast of the United States: Evidence of Divergence in the Abundance of Two Sympatric Phocid Species? PLoS ONE 2015, 10, e0131660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- COSEWIC. Status Appraisal Summary. 2018. Available online: https://www.canada.ca/en/environment-climate-change/services/species-risk-public-registry/cosewic-assessments-status-reports/harbour-seal-2018.html (accessed on 6 May 2019).
- Teilmann, J.; Dietz, R. Status of the harbour seal, Phoca vitulina, in Greenland. Oceanogr. Lit. Rev. 1995, 42, 566. [Google Scholar]
- Wiig, Ø. A description of common seals (Phoca vitulina L. 1758 from Svalbard. Mar. Mammal Sci. 1989, 5, 149–158. [Google Scholar] [CrossRef]
- Lydersen, C.; Kovacs, K.M. Growth and population parameters of the world’s northernmost harbour seals Phoca vitulina residing in Svalbard, Norway. Polar Biol. 2005, 28, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, G.; Gjertz, I.; Kondakov, A. A review of the distribution and abundance of harbor seals, Phoca vitulina, on Svalbard, Norway, and in the Barents Sea. Mar. Mammal Sci. 1997, 13, 157–163. [Google Scholar] [CrossRef]
- Blanchet, M.-A.; Lydersen, C.; Ims, R.A.; Kovacs, K.M. Making it through the first year: Ontogeny of movement and diving behavior in harbor seals from Svalbard, Norway. Mar. Mammal Sci. 2016, 32, 1340–1369. [Google Scholar] [CrossRef]
- Blanchet, M.-A.; Lydersen, C.; Ims, R.A.; Kovacs, K.M. Seasonal, oceanographic and atmospheric drivers of diving behaviour in a temperate seal species living in the high arctic. PLoS ONE 2015, 10, e0132686. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, C.D.; Lydersen, C.; Ims, R.A.; Kovacs, K.M. Haul-Out Behaviour of the World’s Northernmost Population of Harbour Seals (Phoca vitulina) throughout the Year. PLoS ONE 2014, 4, e86055. [Google Scholar] [CrossRef]
- Leclerc, L.-M.E.; Lydersen, C.; Haug, T.; Bachmann, L.; Fisk, A.T.; Kovacs, K.M. A missing piece in the Arctic food web puzzle? Stomach contents of Greenland sharks sampled in Svalbard, Norway. Polar Biol. 1197, 2012, 35–1208. [Google Scholar] [CrossRef]
- Routti, H.; Lydersen, C.; Hanssen, L.; Kovacs, K.M. Contaminant levels in the world’s northernmost harbor seals (Phoca vitulina). Mar. Pollut. Bull. 2014, 1–7. [Google Scholar] [CrossRef]
- Hauksson, E.; Einarsson, S.T. Historical trend in harbour seal (Phoca vitulina) abundance in Iceland back to the year 1912. NAMMCO Sci. Publ. 2010, 8, 147. [Google Scholar] [CrossRef] [Green Version]
- Granquist, S.M.; Hauksson, E. Seasonal, meteorological, tidal and diurnal effects on haul-out patterns of harbour seals (Phoca vitulina) in Iceland. Polar Biol. 2016, 39, 2347–2359. [Google Scholar] [CrossRef]
- Hauksson, E. Sealing in Iceland in 1982–1989. Hafrannsoknir 1992, 43, 59–70. [Google Scholar]
- Granquist, S.M.; Esparza-Salas, R.; Hauksson, E.; Karlsson, O.; Angerbjörn, A. Fish consumption of harbour seals (Phoca vitulina) in north western Iceland assessed by DNA metabarcoding and morphological analysis. Polar Biol. 2018, 41, 2199–2210. [Google Scholar] [CrossRef]
- Punt, A.E.; Siple, M.; Sigurðsson, G.M.; Víkingsson, G.; Francis, T.B.; Granquist, S.M.; Hammond, P.S.; Heinemann, D.; Long, K.J.; Moore, J.E.; et al. Evaluating management strategies for marine mammal populations: An example for multiple species and multiple fishing sectors in Iceland. Can. J. Fish. Aquat. Sci. 2020, 77, 1316–1331. [Google Scholar] [CrossRef]
- Þorbjornsson, J.G.; Hauksson, E.; Sigurðsson, G.M.; Granquist, S.M. Aerial Census of the Icelandic Harbour Seal Population in 2016: Population Estimate, Trends and Current Status. 2017. Available online: https://www.hafogvatn.is/is/midlun/utgafa/haf-og-vatnarannsoknir/aerial-census-of-the-icelandic-harbour-seal-phoca-vitulina-population-in-2016-population-estimate-trends-and-current-status-landselstalning-2016-stofnstaerdarmat-sveiflur-og-astand-stofns (accessed on 8 May 2019).
- Granquist, S.M.; Hauksson, E. Population Estimate, Trends and Current Status of the Icelandic Harbour Seal (Phoca vitulina) Population in 2018. Marine and Freshwater Research in Iceland. HV 2019-36. 2019. Available online: https://www.hafogvatn.is/static/files/hv2019-36.pdf (accessed on 8 May 2019).
- Bloch, D.; Mikkelsen, B.; Ofstad, L.H. Marine Mammals in Faroese Waters-With Special Attention to the South-South-Eastern Sector of the Region. 2000. Available online: http://projects.foib.fo/eia/Faroe_eia/Studies/Mammal_Final_Part1.pdf (accessed on 8 May 2019).
- Mikkelsen, B. A note on the harbour seal (Phoca vitulina) in the Faroe Islands. NAMMCO Sci. Publ. 2010, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Bjørge, A. Status of the harbour seal Phoca vitulina L. in Norway. Biol. Conserv. 1991, 58, 229–238. [Google Scholar] [CrossRef]
- Hassani, S.; Dupuis, L.; Elder, J.F.; Caillot, E.; Gautier, G.; Hemon, A.; Lair, J.M.; Haelters, J. A note on harbour seal (Phoca vitulina) distribution and abundance in France and Belgium. NAMMCO Sci. Publ. 2010, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Heide-Jørgensen, M.-P.; Härkönen, T.J. Rebuilding seal stocks in the Kattegat-Skagerrak. Mar. Mammal Sci. 1988, 4, 231–246. [Google Scholar] [CrossRef]
- Nilssen, K.T.; Skavberg, N.-E.; Poltermann, M.; Haug, T.; Härkönen, T.; Henriksen, G. Status of harbour seals (Phoca vitulina) in mainland Norway. NAMMCO Sci. Publ. 2010, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Härkönen, T.; Dietz, R.; Reijnders, P.; Teilmann, J.; Harding, K.; Hall, A.; Brasseur, S.; Siebert, U.; Goodman, S.J.; Jepson, P.D.; et al. The 1988 and 2002 phocine distemper virus epidemics in European harbour seals. Dis. Aquat. Organ. 2006, 68, 115–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zyryanov, S.V.; Egorov, S.A. Status of the harbour seal (Phoca vitulina) along the Murman coast of Russia. NAMMCO Sci. Publ. 2010, 8, 37. [Google Scholar] [CrossRef]
- Mishin, V.L.; Zyryanov, S.V.; Goryaev, Y.I. Preliminary insight into the harbour seal population of the east Murmansk coast of the Barents Sea. Mammalia 2001, 65, 534–540. [Google Scholar] [CrossRef]
- NAMMCO. Report of the NAMMCO Scientific Committee Meeting 4–7 November 2016. 2016. Available online: https://nammco.no/topics/scientific-committee-reports/ (accessed on 10 May 2019).
- HELCOM. Population Trends and Abundance of Seals. HELCOM Core Indicator Report. 2018. Available online: https://www.helcom.fi/wp-content/uploads/2019/08/Population-trends-and-abundance-of-seals-HELCOM-core-indicator-2018.pdf (accessed on 10 May 2019).
- Olsen, M.T.; Andersen, S.M.; Teilmann, J.; Dietz, R.; Edrén, S.M.C.; Linnet, A.; Härkönen, T. Status of the harbour seal (Phoca vitulina) in Southern Scandinavia. NAMMCO Sci. Publ. 2010, 8. [Google Scholar] [CrossRef] [Green Version]
- Harkonen, T.; Andersen, S.M.; Teilmann, J.; Dietz, R.; Edrén, S.M.; Linnet, A.; Härkönen, T. Mass mortality in harbour seals and harbour porpoises caused by an unknown pathogen. Vet. Rec. 2008, 162, 555–556. [Google Scholar] [CrossRef] [Green Version]
- Zohari, S.; Neimanis, A.; Härkönen, T.; Moraeus, C.; Valarcher, J.F. Avian influenza A(H10N7) virus involvement in mass mortality of harbour seals (Phoca vitulina) in Sweden, March through October 2014. Euro Surveill. 2014, 19. [Google Scholar] [CrossRef]
- Olsson, M.; Karlsson, B.; Ahnland, E. Diseases and environmental contaminants in seals from the Baltic and the Swedish west coast. Sci. Total Environ. 1994, 154, 217–227. [Google Scholar] [CrossRef]
- Härkönen, T.; Isakson, E. Status of harbour seals (Phoca vitulina) in the Baltic proper. NAMMCO Sci. Publ. 2010, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Bjurlid, F.; Roos, A.; Jogsten, I.E.; Hagberg, J. Temporal trends of PBDD/Fs, PCDD/Fs, PBDEs and PCBs in ringed seals from the Baltic Sea (Pusa hispida botnica) between 1974 and 2015. Sci. Total Environ. 2018, 616, 1374–1383. [Google Scholar] [CrossRef]
- Galatius, A.; Brasseur, S.M.J.M.; Busch, J.A.; Cremer, J.S.M.; Czeck, R.; Jeß, A.; Diederichs, B.; Körber, P.; Pund, R.; Siebert, U.; et al. Trilateral surveys of Harbour Seals in the Wadden Sea and Helgoland in 2019. Available online: https://www.waddensea-secretariat.org/resources/2019-harbour-seal-report (accessed on 8 May 2019).
- Tougaard, J.; Henriksen, O.D.; Miller, L.A. Underwater noise from three types of offshore wind turbines: Estimation of impact zones for harbor porpoises and harbor seals. J. Acoust. Soc. Am. 2009, 3766, 125–3773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, C.; Huon, M.; Caurant, F.; Dabin, W.; Deniau, A.; Dixneuf, S.; Dupuis, L.; Elder, J.F.; Fremau, M.H.; Hassani, S.; et al. Grey and harbour seals in France: Distribution at sea, connectivity and trends in abundance at haul-out sites. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.; Duck, C.D.; Morris, C.D.; Russell, D.J.F. The status of harbour seals (Phoca vitulina) in the UK. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 40–60. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.; Duck, C.; Lonergan, M.E. The status of harbour seals (Phoca vitulina) in the United Kingdom. NAMMCO Sci. Publ. 2010, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Lonergan, M.; Duck, C.; Moss, S.; Morris, C.; Thompson, D. Rescaling of aerial survey data with information from small numbers of telemetry tags to estimate the size of a declining harbour seal population. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 135–144. [Google Scholar] [CrossRef]
- Lonergan, M.; Duck, C.D.; Thompson, D.; Mackey, B.L.; Cunningham, L.; Boyd, I.L. Using sparse survey data to investigate the declining abundance of British harbour seals. J. Zool. 2007, 271, 261–269. [Google Scholar] [CrossRef]
- Wilson, L.J.; Hammond, P.S. The diet of harbour and grey seals around Britain: Examining the role of prey as a potential cause of harbour seal declines. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.L.; Sparling, C.E.; McConnell, B.J.; Morris, C.D.; Smout, S. Fine-scale harbour seal usage for informed marine spatial planning. Sci. Rep. 2017, 7, 11581. [Google Scholar] [CrossRef] [Green Version]
- Boyd, I.L. Scientific Advice on Matters Related to the Management of Seal Populations: 2002. Natural Environment Research Council. Available online: http://www.smru.st-and.ac.uk/CurrentResearch.htm/scos.htm (accessed on 6 May 2019).
- Jensen, S.K.; Lacaze, J.P.; Hermann, G.; Kershaw, J.; Brownlow, A.; Turner, A.; Hall, A. Detection and effects of harmful algal toxins in Scottish harbour seals and potential links to population decline. Toxicon 2015, 97, 1–14. [Google Scholar] [CrossRef]
- Cronin, M.A. The status of the harbour seal (Phoca vitulina) in Ireland. NAMMCO Sci. Publ. 2010, 8. [Google Scholar] [CrossRef]
- Kavanagh, A.S.; Cronin, M.A.; Walton, M.; Rogan, E. Diet of the harbour seal (Phoca vitulina vitulina) in the west and south-west of Ireland. J. Mar. Biol. Assoc. U. K. 2010, 90, 1517–1527. [Google Scholar] [CrossRef]
- Cronin, M.; Duck, C.; Cadhla, O.Ó.; Nairn, R.; Strong, D.; O’Keeffe, C. An assessment of population size and distribution of harbour seals in the Republic of Ireland during the moult season in August 2003. J. Zool. 2007, 273, 131–139. [Google Scholar] [CrossRef]
- Cronin, M.; Gregory, S.; Rogan, E. Moulting phenology of the harbour seal in south-west Ireland. J. Mar. Biol. Assoc. U. K. 2014, 1079, 94–1086. [Google Scholar] [CrossRef] [Green Version]
- Luck, C.; Cronin, M.; Gosch, M.; Healy, K.; Cosgrove, R.; Tully, O.; Rogan, E.; Jessopp, M. Drivers of spatiotemporal variability in bycatch of a top marine predator: First evidence for the role of water turbidity in protected species bycatch. J. Appl. Ecol. 2020, 57, 219–228. [Google Scholar] [CrossRef]
- Cosgrove, R.; Gosch, M.; Reid, D.; Sheridan, M.; Chopin, N.; Jessopp, M.; Cronin, M. Seal bycatch in gillnet and entangling net fisheries in Irish waters. Fish. Res. 2016, 183, 192–199. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information. State of the Climate: Global Climate Report for August 2019. Available online: https://www.ncdc.noaa.gov/sotc/global/201908 (accessed on 31 December 2019).
- Hansen, B.B.; Isaksen, K.; Benestad, R.E.; Kohler, J.; Larsen, J.O.; Varpe, Ø. Warmer and wetter winters: Characteristics and implications of an extreme weather event in the High Arctic. Environ. Res. Lett. 2014, 9, 114021. [Google Scholar] [CrossRef]
- Simpkins, M.A.; Withrow, D.E.; Cesarone, J.C.; Boveng, P.L. Stability in the proportion of harbor seals hauled out under locally ideal conditions. Mar. Mammal Sci. 2003, 19, 791–805. [Google Scholar] [CrossRef]
- Godsell, J. Herd formation and haul-out behaviour in harbour seals (Phoca vitulina). J. Zool. 1988, 215, 83–98. [Google Scholar] [CrossRef]
- Hansen, S.; Lavigne, D.M. Ontogeny of the Thermal Limits in the Harbor Seal (Phoca vitulina) Ontogeny. Physiol. Zool. 1997, 70, 85–92. [Google Scholar] [CrossRef]
- Comiso, J.C.; Hall, D.K. Climate trends in the Arctic as observed from space. Wiley Interdiscip. Rev. Clim. Chang. 2014, 5, 389–409. [Google Scholar] [CrossRef]
- Maslanik, J.A.; Fowler, C.; Stroeve, J.; Drobot, S.; Zwally, J.; Yi, D.; Emery, W. A younger, thinner Arctic ice cover: Increased potential for rapid, extensive sea-ice loss. Geophys. Res. Lett. 2004, 2007, 34–2008. [Google Scholar] [CrossRef] [Green Version]
- Bajzak, C.E.; Bernhardt, W.; Mosnier, A.; Hammill, M.O.; Stirling, I. Habitat use by harbour seals (Phoca vitulina) in a seasonally ice-covered region, the western Hudson Bay. Polar Biol. 2013, 36, 477–491. [Google Scholar] [CrossRef]
- Lesage, V.; Hammill, M.O.; Kovacs, K.M. Long-distance movements of harbour seals (Phoca vitulina) from a seasonally ice-covered area, the St. Lawrence River estuary, Canada. Can. J. Zool. 2004, 1070, 82–1081. [Google Scholar] [CrossRef]
- Florko, K.R.N. Decreasing sea ice conditions in western Hudson Bay and an increase in abundance of harbour seals (Phoca vitulina) in the Churchill River. Polar Biol. 2018, 1187, 41–1195. [Google Scholar] [CrossRef]
- Baechler, J.; Beck, C.A.; Bowen, W.D. Dive shapes reveal temporal changes in the foraging behaviour of different age and sex classes of harbour seals (Phoca vitulina). Can. J. Zool. 2002, 1569, 80–1577. [Google Scholar] [CrossRef] [Green Version]
- Ramasco, V.; Barraquand, F.; Biuw, M.; McConnell, B.; Nilssen, K.T. The intensity of horizontal and vertical search in a diving forager: The harbour seal. Mov. Ecol. 2015, 3, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Zemp, M.; Huss, M.; Thibert, E.; Eckert, N.; McNabb, R.; Huber, J.; Barandun, M.; Machguth, H.; Nussbaumer, S.U.; Gärtner-Roer, I.; et al. Global glacier mass changes and their contributions to sea-level rise from 1961 to 2016. Nature 2019, 568, 382–386. [Google Scholar] [CrossRef]
- Wouters, B.; Gardner, A.S.; Moholdt, G. Global Glacier Mass Loss During the GRACE Satellite Mission (2002–2016). Front. Earth Sci. 2019, 7, 96. [Google Scholar] [CrossRef] [Green Version]
- Fay, F.H. The role of ice in the ecology of marine mammals of the Bering Sea. Oceanogr. Bering Sea 1974, 2, 383–399. [Google Scholar]
- Blundell, G.M.; Womble, J.N.; Pendleton, G.W.; Karpovich, S.A.; Gende, S.M.; Herreman, J.K. Use of glacial and terrestrial habitats by harbor seals in Glacier Bay, Alaska: Costs and benefits. Mar. Ecol. Prog. Ser. 2011, 429, 277–290. [Google Scholar] [CrossRef]
- Lydersen, C.; Vaquie-Garcia, J.; Lydersen, E.; Christensen, G.N.; Kovacs, K.M. Novel terrestrial haul-out behaviour by ringed seals (Pusa hispida) in Svalbard, in association with harbour seals (Phoca vitulina). Polar Res. 2017, 36, 1374124. [Google Scholar] [CrossRef] [Green Version]
- Cordes, L.S.; O’Corry-Crowe, G.; Small, R.J. Surreptitious sympatry: Exploring the ecological and genetic separation of two sibling species. Ecol. Evol. 2017, 1725, 7–1736. [Google Scholar] [CrossRef] [PubMed]
- Trillmich, F.; Dellinger, T. The effects of El Niño on Galapagos pinnipeds. In Pinnipeds and El Niño Ecological Studies (Analysis and Synthesis); Trillmich, F., Ono, K.A., Eds.; Springer: Berlin, Heidelberg, 1991; pp. 66–74. [Google Scholar]
- Lusseau, D.; Williams, R.; Wilson, B.; Grellier, K.; Barton, T.R.; Hammond, P.S.; Thompson, P.M. Parallel influence of climate on the behaviour of Pacific killer whales and Atlantic bottlenose dolphins. Ecol. Lett. 2004, 1068, 7–1076. [Google Scholar] [CrossRef]
- Friedlaender, A.S.; Johnston, D.W.; Halpin, P.N. Progress in Oceanography Effects of the North Atlantic Oscillation on sea ice breeding habitats of harp seals (Pagophilus groenlandicus) across the North Atlantic. Prog. Oceanogr. 2010, 86, 261–266. [Google Scholar] [CrossRef]
- Trites, A.W.; Donnelly, C.P. The decline of Steller sea lions Eumetopias jubatus in Alaska: A review of the nutritional stress hypothesis. Mammals Rev. 2003, 33, 3–28. [Google Scholar] [CrossRef] [Green Version]
- Steingass, S.; Horning, M. Individual-based energetic model suggests bottom up mechanisms for the impact of coastal hypoxia on Pacific harbor seal (Phoca vitulina richardii) foraging behavior. J. Theor. Biol. 2017, 416, 190–198. [Google Scholar] [CrossRef]
- Steingass, S.M.; Naito, Y. Ocean Deoxygenation: Everyone’s Problem. Causes, Impacts, Consequences and Solutions. 2020. Available online: https://portals.iucn.org/library/sites/library/files/documents/08.10DEOX.pdf (accessed on 30 December 2020).
- Fossheim, M.; Primicerio, R.; Johannesen, E.; Ingvaldsen, R.B.; Aschan, M.M.; Dolgov, A.V. Recent warming leads to a rapid borealization of fish communities in the Arctic. Nat. Clim. Chang. 2015, 5, 673. [Google Scholar] [CrossRef]
- Kortsch, S.; Primicerio, R.; Aschan, M.; Lind, S.; Dolgov, A.V.; Planque, B. Food-web structure varies along environmental gradients in a high-latitude marine ecosystem. Ecography 2019, 42, 295–308. [Google Scholar] [CrossRef]
- Frainer, A.; Primicerio, R.; Kortsch, S.; Aune, M.; Dolgov, A.V.; Fossheim, M.; Aschan, M.M. Climate-driven changes in functional biogeography of Arctic marine fish communities. Proc. Natl. Acad. Sci. USA 2017, 114, 12202–12207. [Google Scholar] [CrossRef] [Green Version]
- Randelhoff, A.; Reigstad, M.; Chierici, M.; Sundfjord, A.; Ivanov, V.; Cape, M.; Vernet, M.; Tremblay, J.É.; Bratbak, G.; Kristiansen, S. Seasonality of the Physical and Biogeochemical Hydrography in the Inflow to the Arctic Ocean Through Fram Strait. Front. Marine Sci. 2018, 5, 224. [Google Scholar] [CrossRef] [Green Version]
- Ingvaldsen, R.B.; Gjøsæter, H.; Ona, E.; Michalsen, K. Atlantic cod (Gadus morhua) feeding over deep water in the high Arctic. Polar Biol. 2017, 40, 2105–2111. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.M.; Lydersen, C.; Grahl-Nielsen, O.; Kovacs, K.M. Autumn diet of harbour seals (Phoca vitulina) at Prins Karls Forland, Svalbard, assessed via scat and fatty-acid analyses. Can. J. Zool. 2004, 1230, 82–1245. [Google Scholar] [CrossRef] [Green Version]
- Colominas, R. Harbour Seal Diet in a Changing Arctic (Svalbard, Norway). Master’s Thesis, University of Bergen, Bergen, Norway, 2012. [Google Scholar]
- Thompson, P.M.; Mcconnell, B.J.; Tollit, D.J.; Mackay, A.; Hunter, C.; Racey, P.A. Comparative Distribution, Movements and Diet of Harbour and Grey Seals from Moray Firth, N.E. Scotland. J. Appl. Ecol. 1996, 1572, 33–1584. [Google Scholar] [CrossRef]
- Thompson, P.M.; Tollit, D.J.; Corpe, H.M.; Reid, R.J.; Ross, H.M. Changes in haematological parameters in relation to prey switching in a wild population of harbour seals. Funct. Ecol. 1997, 11, 743–750. [Google Scholar] [CrossRef]
- Weijerman, M.; Lindeboom, H.; Zuur, A.F. Regime shifts in marine ecosystems of the North Sea and Wadden Sea. Mar. Ecol. Prog. Ser. 2005, 298, 21–39. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.C.; Peterson, G.; Bodin, Ö.; Levin, S. Cascading regime shifts within and across scales. Science 2018, 362, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, M.-A.; Primicerio, R.; Frainer, A.; Kortsch, S.; Skern-Mauritzen, M.; Dolgov, A.V.; Aschan, M. The role of marine mammals in the Barents Sea foodweb. ICES J. Mar. Sci. 2019, 76, i37–i53. [Google Scholar] [CrossRef]
- Ferguson, S.H.; Higdon, J.W.; Chmelnitsky, E.W. The Rise of Killer Whales as a Major Arctic Predator. In A Little Less Arctic; Ferguson, S.H., Loseto, L.L., Mallory, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Higdon, J.W.; Hauser, D.D.W.; Ferguson, S.H. Killer whales (Orcinus orca) in the Canadian Arctic: Distribution, prey items, group sizes, and seasonality. Mar. Mammal Sci. 2012, 28, E93–E109. [Google Scholar] [CrossRef]
- Tryland, M.; Godfroid, J.; Arneberg, P. Impact of Climate Change on Infectious Diseases of Animals in the Norwegian Arctic. 2009. Norwegian Polar Institute Brief Report Series. Available online: https://brage.npolar.no/npolar-xmlui/handle/11250/172981 (accessed on 30 December 2020).
- VanWormer, E.; Mazet, J.A.; Hall, A.; Gill, V.A.; Boveng, P.L.; London, J.M.; Gelatt, T.; Fadely, B.S.; Lander, M.E.; Sterling, J.; et al. Viral emergence in marine mammals in the North Pacific may be linked to Arctic sea ice reduction. Sci. Rep. 2019, 9, 15569. [Google Scholar] [CrossRef] [Green Version]
- Polley, L.; Thompson, R.C.A. Parasite zoonoses and climate change: Molecular tools for tracking shifting boundaries. Trends Parasitol. 2009, 25, 285–291. [Google Scholar] [CrossRef]
- Brooks, D.R.; Hoberg, E.P. How will global climate change affect parasite-host assemblages? Trends Parasitol. 2007, 23, 571–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, M.J.; Kutz, S.J.; Jenkins, E.; O’Hara, T.M. The potential impact of climate change on infectious diseases of Arctic fauna. Int. J. Circumpolar Health 2005, 64, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Keroack, C.D.; Williams, K.M.; Fessler, M.K.; DeAngelis, K.E.; Tsekitsidou, E.; Tozloski, J.M.; Williams, S.A. A novel quantitative real-time PCR diagnostic assay for seal heartworm (Acanthocheilonema spirocauda) provides evidence for possible infection in the grey seal (Halichoerus grypus). Int. J. Parasitol. Parasites Wildl. 2018, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, K.; Schwanke, E.; Hahn, K.; Wohlsein, P.; Siebert, U. Heartworm (Acanthocheilonema spirocauda) and seal louse (Echinophthirius horridus) infections in harbour seals (Phoca vitulina) from the North and Baltic Seas. J. Sea Res. 2016, 113, 65–72. [Google Scholar] [CrossRef]
- Burek, K.A.; Gulland, F.M.D.; O’Hara, T.M. Effects of climate change on arctic marine mammal health. Ecol. Appl. 2008, 18, S126–S134. [Google Scholar] [CrossRef]
- Galaktionov, K.V. Patterns and processes influencing helminth parasites of Arctic coastal communities during climate change. J. Helminthol. 2017, 91, 387–408. [Google Scholar] [CrossRef]
- Harvell, C.D.; Kim, K.; Burkholder, J.M.; Colwell, R.R.; Epstein, P.R.; Grimes, D.J.; Hofmann, E.E.; Lipp, E.K.; Osterhaus, A.D.; Overstreet, R.M.; et al. Emerging marine diseases—Climate links and anthropogenic factors. Science 1999, 1505, 285–1510. [Google Scholar] [CrossRef] [Green Version]
- Marcogliese, D.J. Implications of climate change for parasitism of animals in the aquatic environment. Can. J. Zool. 2001, 79, 1331–1352. [Google Scholar] [CrossRef]
- Siebert, U.; Gulland, F.; Harder, T.; Jauniaux, T.; Seibel, H.; Wohlsein, P.; Baumgärtner, W. Epizootics in harbour seals (Phoca vitulina): Clinical aspects. NAMMCO Sci. Publ. 2010, 8, 265. [Google Scholar] [CrossRef]
- Stokholm, I.; Härkönen, T.; Harding, K.C.; Siebert, U.; Lehnert, K.; Dietz, R.; Teilmann, J.; Galatius, A.; Havmøller, L.W.; Carroll, E.L.; et al. Phylogenomic insights to the origin and spread of phocine distemper virus in European harbour seals in 1988 and 2002. Dis. Aquat. Organ. 2019, 133, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Lavigne, D.M.; Schmitz, O.J. Global warming and increasing population densities: A prescription for seal plagues. Mar. Pollut. Bull. 1990, 21, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Rosing-Asvid, A.; Teilmann, J.; Dietz, R.; Olsen, M. First Confirmed Record of Grey Seals in Greenland. Arctic 2010, 63, 471–473. [Google Scholar] [CrossRef] [Green Version]
- Sommer, S. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front. Zool. 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, K.; Kobayashi, M.; Kai, C. Seroepidemiological survey of morbillivirus infection in Kuril harbor seals Phoca vitulina stejnegeri of Hokkaido, Japan. Jpn. J. Vet. Res. 2006, 54, 109–117. [Google Scholar] [PubMed]
- Goldstein, T.; Mazet, J.A.K.; Gill, V.A.; Doroff, A.M.; Burek, K.A.; Hammond, J.A. Phocine distemper virus in northern sea otters in the Pacific Ocean, Alaska, USA. Emerg. Infect. Dis. 2009, 15, 925–927. [Google Scholar] [CrossRef]
- Sanderson, C.E.; Alexander, K.A. Unchartered waters: Climate change likely to intensify infectious disease outbreaks causing mass mortality events in marine mammals. Glob. Chang. Biol. 2020, 26, 4284–4301. [Google Scholar] [CrossRef]
- Jensen, S.K.; Aars, J.; Lydersen, C.; Kovacs, K.M.; Åsbakk, K. The prevalence of Toxoplasma gondii in polar bears and their marine mammal prey: Evidence for a marine transmission pathway? Polar Biol. 2010, 33, 599–606. [Google Scholar] [CrossRef]
- Gibson, A.K.; Raverty, S.; Lambourn, D.M.; Huggins, J.; Magargal, S.L.; Grigg, M.E. Polyparasitism is associated with increased disease severity in Toxoplasma gondii-infected marine sentinel species. PLoS Negl. Trop. Dis. 2011, 5, e1142. [Google Scholar] [CrossRef] [Green Version]
- Corbett, J.J.; Lack, D.A.; Winebrake, J.J.; Harder, S.; Silberman, J.A.; Gold, M. Arctic shipping emissions inventories and future scenarios. Atmos. Chem. Phys. 2010, 10, 9689–9704. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.L.; Hastie, G.D.; Smout, S.; Onoufriou, J.; Merchant, N.D.; Brookes, K.L.; Thompson, D. Seals and shipping: Quantifying population risk and individual exposure to vessel noise. J. Appl. Ecol. 2017, 54, 1930–1940. [Google Scholar] [CrossRef]
- Ahrens, L.; Siebert, U.; Ebinghaus, R. Temporal trends of polyfluoroalkyl compounds in harbor seals (Phoca vitulina) from the German Bight, 1999–2008. Chemosphere 2009, 76, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Blévin, P.; Aars, J.; Andersen, M.; Blanchet, M.A.; Hanssen, L.; Herzke, D.; Jeffreys, R.M.; Nordøy, E.S.; Pinzone, M.; de la Vega, C.; et al. Pelagic vs Coastal—Key Drivers of Pollutant Levels in Barents Sea Polar Bears with Contrasted Space-Use Strategies. Environ. Sci. Technol. 2020, 54, 985–995. [Google Scholar] [CrossRef]
- Mos, L.; Morsey, B.; Jeffries, S. Chemical and biological pollution contribute to the immunological profiles of free-ranging harbor seals. Env. Toxicol Chem 2006, 25, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.; Johns, D.G.; Leterme, S.C.; Svendsen, E.; Richardson, A.J. Regional climate change and harmful algal blooms in the northeast Atlantic. Limnol. Oceanogr. 2006, 51, 820–829. [Google Scholar] [CrossRef] [Green Version]
- Harvey, J.T. Phoca vitulina ssp. richardii. The IUCN Red List of Threatened Species 2016. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T17022A66991556.en (accessed on 17 September 2020).
- Temple, H.J.; Terry, A. The Status and Distribution of European Mammals. 2007. Available online: https://ec.europa.eu/environment/nature/conservation/species/redlist/downloads/European_mammals.pdf (accessed on 17 September 2020).
- Matsuda, H.; Yamamura, O.; Kitakado, T.; Kobayashi, Y.; Kobayashi, M.; Hattori, K.; Kato, H. Beyond dichotomy in the protection and management of marine mammals in Japan. Therya 2015, 6, 283–296. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanchet, M.-A.; Vincent, C.; Womble, J.N.; Steingass, S.M.; Desportes, G. Harbour Seals: Population Structure, Status, and Threats in a Rapidly Changing Environment. Oceans 2021, 2, 41-63. https://doi.org/10.3390/oceans2010003
Blanchet M-A, Vincent C, Womble JN, Steingass SM, Desportes G. Harbour Seals: Population Structure, Status, and Threats in a Rapidly Changing Environment. Oceans. 2021; 2(1):41-63. https://doi.org/10.3390/oceans2010003
Chicago/Turabian StyleBlanchet, Marie-Anne, Cécile Vincent, Jamie N. Womble, Sheanna M. Steingass, and Geneviève Desportes. 2021. "Harbour Seals: Population Structure, Status, and Threats in a Rapidly Changing Environment" Oceans 2, no. 1: 41-63. https://doi.org/10.3390/oceans2010003
APA StyleBlanchet, M. -A., Vincent, C., Womble, J. N., Steingass, S. M., & Desportes, G. (2021). Harbour Seals: Population Structure, Status, and Threats in a Rapidly Changing Environment. Oceans, 2(1), 41-63. https://doi.org/10.3390/oceans2010003