Larval Fish Community in the Northwestern Iberian Upwelling System during the Summer Period
Abstract
:1. Introduction
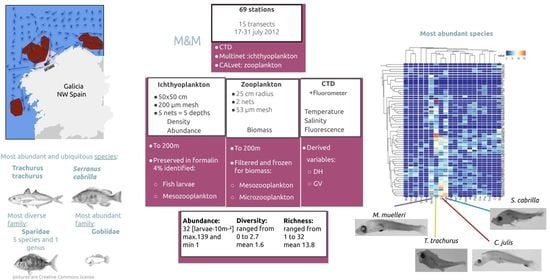
2. Materials and Methods
2.1. Environmental Data
2.2. Community
2.3. Horizontal Distribution
2.4. Vertical Distribution
3. Results
3.1. Environmental Variables
3.2. Biological Variables
3.3. Descriptors and Structure of the Larval Fish Community
3.4. Relationship between the LFC and Environmental Variables
4. Discussion
4.1. Environmental Conditions and Their Influence on the Summer LFC
4.2. Seasonal LFC in Galician Waters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowen, R.K.; Sponaugle, S. Larval Dispersal and Marine Population Connectivity. Annu. Rev. Mar. Sci. 2009, 1, 443–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, R.; Barton, E.D.; Miller, P.; Fanjul, E. Spatial patterns of wind and sea surface temperature in the Galician upwelling region. J. Geophys. Res. Space Phys. 2003, 108, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fraga, F. Upwelling off the Galician Coast, Northwest Spain. In Coastal Upwelling; Richards, F.A., Ed.; American Geophysical Union (AGU): Washington, WA, USA, 1981; pp. 176–182. [Google Scholar]
- González-Nuevo, G.; Nogueira, E. Intrusions of warm and salty waters onto the NW and N Iberian shelf in early spring and its relationship to climate variability. J. Atmos. Ocean Sci. 2005, 10, 361–375. [Google Scholar] [CrossRef]
- Alvarez-Salgado, X.A.; Figueiras, F.G.; Perez, F.F.; Groom, S.; Nogueira, E.; Borges, A.V.; Chou, L.; Castro, C.G.; Moncoiffé, G.; Ríos, A.; et al. The Portugal coastal counter current off NW Spain: New insights on its biogeochemical variability. Prog. Oceanogr. 2003, 56, 281–321. [Google Scholar] [CrossRef] [Green Version]
- Ríos, A.F.; Pérez, F.F.; Fraga, F. Water masses in the upper and middle North Atlantic Ocean east of the Azores. Deep Sea Res. Part A Oceanogr. Res. Pap. 1992, 39, 645–658. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.; Barton, E.D. A poleward flow along the Atlantic coast of the Iberian peninsula. J. Geophys. Res. Space Phys. 1990, 95, 11425–11441. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, I.; Prego, R.; de Castro, M.; Varela, M. Galicia upwelling revisited: Out-of-season events in the rias (1967–2009)|Revisión de los eventos de afloramiento en Galicia: Eventos fuera de temporada en las rías (1967–2009). Ciencias Mar. 2012, 38, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Casabella, N.; Lorenzo, M.; Taboada, J. Trends of the Galician upwelling in the context of climate change. J. Sea Res. 2014, 93, 23–27. [Google Scholar] [CrossRef]
- Prego, R.; Bao, R. Upwelling influence on the Galician coast: Silicate in shelf water and underlying surface sediments. Cont. Shelf Res. 1997, 17, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Somoza, L.; Medialdea, T.; González, J.; León, R.; Palomino, D.; Rengel, J.; Fernández-Salas, L.M.; Vazquez, J.-T. Morphostructure of the Galicia continental margin and adjacent deep ocean floor: From hyperextended rifted to convergent margin styles. Mar. Geol. 2018, 407, 299–315. [Google Scholar] [CrossRef]
- Bode, A.; Alvarez-Ossorio, M.; Cabanas, J.; Miranda, A.; Varela, M. Recent trends in plankton and upwelling intensity off Galicia (NW Spain). Prog. Oceanogr. 2009, 83, 342–350. [Google Scholar] [CrossRef]
- Álvarez-Salgado, X.A.; Labarta, U.; Fernández-Reiriz, M.J.; Figueiras, F.G.; Rosón, G.; Piedracoba, S.; Filgueira, R.; Cabanas, J.M. Renewal time and the impact of harmful algal blooms on the extensive mussel raft culture of the Iberian coastal upwelling system (NE Europe). Harmful Algae 2008, 7, 849–855. [Google Scholar]
- Ruiz-Villarreal, M.; Álvarez-Salgado, X.A.; Cabanas, J.M.; Pérez, F.F.; Castro, C.G.; Herrera, J.L.; Piedracoba, S.; Rosón, G. Variabilidade climática e tendencias decadáis nos forzamentos meterorolóxicos e as propiedades das augas adxacentes a Galicia. In Evidencias e Impactos do Cambio Climáticoen Galicia; de Medio Ambiente, C., Ed.; Xunta de Galicia: Galicia, Spain, 2009; pp. 271–286. [Google Scholar]
- Tenore, K.R.; Alonso-Noval, M.; Alvarez-Ossorio, M.; Atkinson, L.P.; Cabanas, J.M.; Cal, R.M.; Campos, H.J.; Castillejo, F.; Chesney, E.J.; Gonzalez, N.; et al. Fisheries and oceanography off Galicia, NW Spain: Mesoscale spatial and temporal changes in physical processes and resultant patterns of biological productivity. J. Geophys. Res. Space Phys. 1995, 100, 10943–10966. [Google Scholar] [CrossRef]
- Fariña, A.; Freire, J.; González-Gurriarán, E. Demersal Fish Assemblages in the Galician Continental Shelf and Upper Slope (NW Spain): Spatial Structure and Long-term Changes. Estuar. Coast. Shelf Sci. 1997, 44, 435–454. [Google Scholar] [CrossRef]
- Santos, M.B.; González-Quirós, R.; Riveiro, I.; Iglesias, M.; Louzao, M.; Pierce, G.J. Characterization of the pelagic fish community of the north-western and northern Spanish shelf watersa. J. Fish Biol. 2013, 83, 716–738. [Google Scholar] [CrossRef]
- Ferreiro, M.; Labarta, U. Distribution and abundance of teleostean eggs and larvae on the NW coast of Spain. Mar. Ecol. Prog. Ser. 1988, 43, 189–199. [Google Scholar] [CrossRef]
- Rodríguez, J. Temporal and cross-shelf distribution of ichthyoplankton in the central Cantabrian Sea. Estuar. Coast. Shelf Sci. 2008, 79, 496–506. [Google Scholar] [CrossRef]
- Azeiteiro, U.M.; Bacelar-Nicolau, L.; Resende, P.; Gonçalves, F.J.M.; Pereira, M.J. Larval fish distribution in shallow coastal waters off North Western Iberia (NE Atlantic). Estuar. Coast. Shelf Sci. 2006, 69, 554–566. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.M.; Gonzalez-Pola, C.; Lopez-Urrutia, A.; Nogueira, E. Composition and daytime vertical distribution of the ichthyoplankton assemblage in the central cantabrian Sea shelf, during summer: An eulerian study. Cont. Shelf Res. 2011, 31, 1462–1473. [Google Scholar] [CrossRef]
- Rodriguez, J.; González-Nuevo, G.; Gonzalez-Pola, C.; Cabal, J. The ichthyoplankton assemblage and the environmental variables off the NW and N Iberian Peninsula coasts, in early spring. Cont. Shelf Res. 2009, 29, 1145–1156. [Google Scholar] [CrossRef]
- Rodriguez, J.M. Assemblage structure of ichthyoplankton in the NE Atlantic in spring under contrasting hydrographic conditions. Sci. Rep. 2019, 9, 8636. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, J.M.; Cabrero, A.; Gago, J.; Guevara-Fletcher, C.; Herrero, M.; Hernandez de Rojas, A.; García-García, A.; Laiz-Carrión, R.; Vergara, A.; Alvarez, P.L.; et al. Vertical distribution and migration of fish larvae in the NW Iberian upwelling system during the winter mixing period: Implications for cross-shelf distribution. Fish. Oceanogr. 2015, 24, 274–290. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Cabrero, A.; Gago, J.; Garcia, A.; Laiz-Carrion, R.; Piñeiro, C.; Saborido-Rey, F. Composition and structure of fish larvae community in the NW Iberian upwelling system during the winter mixing period. Mar. Ecol. Prog. Ser. 2015, 533, 245–260. [Google Scholar] [CrossRef] [Green Version]
- García-Seoane, E.; Álvarez-Colombo, G.; Miquel, J.; Rodríguez, J.; Fletcher, C.G.; Álvarez, P.; Saborido-Rey, F. Acoustic detection of larval fish aggregations in Galician waters (NW Spain). Mar. Ecol. Prog. Ser. 2016, 551, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Cowan, J.H., Jr.; Rice, J.C.; Walters, C.J.; Hilborn, R.; Essington, T.E.; Day, J.W., Jr.; Boswell, K.M. Challenges for Implementing an Ecosystem Approach to Fisheries Management. Mar. Coast. Fish. 2012, 4, 496–510. [Google Scholar] [CrossRef]
- Frank, K.T.; Leggett, W.C. Fisheries Ecology in the Context of Ecological and Evolutionary Theory. Annu. Rev. Ecol. Syst. 1994, 25, 401–422. [Google Scholar] [CrossRef]
- Browman, H.I.; Stergiou, K.I. Perspectives on ecosystem-based approaches to the management of marine resources. Mar. Ecol. Prog. Ser. 2004, 274, 269–270. [Google Scholar] [CrossRef] [Green Version]
- Govoni, J.J. Fisheries oceanography and the ecology of early life histories of fishes: A perspective over fifty years. Sci. Mar. 2005, 69, 125–137. [Google Scholar] [CrossRef]
- Lovegrove, T. The Determination of the Dry Weight of Plankton and the Effect of Various Factors on the Values Obtained; Allen and Unwin: St. Leonards, NSW, Australia, 1966. [Google Scholar]
- Bachiller, E.; Fernandes, J.A. Zooplankton Image Analysis Manual: Automated identification by means of scanner and digital camera as imaging devices. Rev. Investig. Mar. 2011, 18, 16–37. [Google Scholar]
- Pond, S.; Pickard, G.L. Introductory Dynamical Oceanography, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1983. [Google Scholar]
- Asch, R.G.; Checkley, D.M. Dynamic height: A key variable for identifying the spawning habitat of small pelagic fishes. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2012, 71, 79–91. [Google Scholar] [CrossRef]
- Siegel, D.A.; McGillicuddy, D.; Fields, E.A. Mesoscale eddies, satellite altimetry, and new production in the Sargasso Sea. J. Geophys. Res. Space Phys. 1999, 104, 13359–13379. [Google Scholar] [CrossRef] [Green Version]
- Lindo-Atichati, D.; Bringas, F.; Goni, G.; Mühling, B.; Muller-Karger, F.; Habtes, S. Varying mesoscale structures influence larval fish distribution in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2012, 463, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Peliz, A.; Rosa, T.L.; Santos, A.M.P.; Pissarra, J.L. Fronts, jets, and counter-flows in the Western Iberian upwelling system. J. Mar. Syst. 2002, 35, 61–77. [Google Scholar] [CrossRef]
- Rhines, P.B. Mesoscale Eddies, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Flament, P. Finestructure and Subduction Associated with Upwelling Filaments; University of California San Diego: La Jolla, CA, USA, 1986. [Google Scholar]
- Flament, P. A state variable for characterizing water masses and their diffusive stability: Spiciness. Prog. Oceanogr. 2002, 54, 493–501. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kelley, D.E. Oceanographic Analysis with R; Springer: New York, NY, USA, 2018. [Google Scholar]
- Instituto Español de Oceanografía. Data Viewer IEO. 2011. Available online: http://www.indicedeafloramiento.ieo.es/index1_en.php (accessed on 12 November 2018).
- Organismo Público Puertos del Estado, Ministerio de Transportes. Puertos del Estado—Oceanografía. Available online: http://www.puertos.es/es-es/oceanografia/ (accessed on 12 November 2018).
- Gräler, B.; Pebesma, E.; Heuvelink, G. Spatio-Temporal Interpolation using gstat. R J. 2016, 8, 204–218. [Google Scholar] [CrossRef]
- Hiemstra, P.H.; Pebesma, E.; Twenhöfel, C.J.; Heuvelink, G.B. Real-time automatic interpolation of ambient gamma dose rates from the Dutch radioactivity monitoring network. Comput. Geosci. 2009, 35, 1711–1721. [Google Scholar] [CrossRef]
- Smith, P.E.; Richardson, S.L. Standard techniques for pelagic, fish egg and larva surveys. FAO Fish. Tech. Pap. 1977, 175, 100. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecologywith R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2009, 1, 3–14. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2010, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Field, J.; Clarke, K.; Warwick, R. A Practical Strategy for Analysing Multispecies Distribution Patterns. Mar. Ecol. Prog. Ser. 1982, 8, 37–52. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E: Plymouth, UK, 2001; p. 170. [Google Scholar]
- Scrucca, L.; Fop, M.; Murphy, T.B.; Raftery, A.E. Mclust 5: Clustering, classification and density estimation using Gaussian finite mixture models. R. J. 2016, 8, 289–317. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J. Constrained Ordination: Tutorial with R and vegan Preliminaries: Inspecting Data. R-Packace Vegan 2012, 1, 1–10. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.0-2. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 6 November 2020).
- Fortier, L.; Leggett, W.C. Vertical Migrations and Transport of Larval Fish in a Partially Mixed Estuary. Can. J. Fish. Aquat. Sci. 1983, 40, 1543–1555. [Google Scholar] [CrossRef]
- Neilson, J.D.; Perry, R.I. Diel vertical migrations of juvenile fish: An obligate or facultative process? Adv. Mar. Biol. 1990, 26, 115–168. [Google Scholar]
- Torres, R.; Barton, E. Onset of the Iberian upwelling along the Galician coast. Cont. Shelf Res. 2007, 27, 1759–1778. [Google Scholar] [CrossRef] [Green Version]
- Somavilla, R.; González-Pola, C.; Fernández-Diaz, J. The warmer the ocean surface, the shallower the mixed layer. Howmuch of this is true? J. Geophys. Res. Ocean. 2017, 122, 7698–7716. [Google Scholar] [CrossRef]
- Fletcher Guevara, C.E. Characterization and Influence of Biotic and Abiotic Factors on the Early Life Stages of European Hake (Merluccius merluccius L. 1758) from the Southern Stock. Ph.D. Thesis, Department of Zoology and Animal Cell Biology, Universidad del País Vasco, Leioa, Spain, 2017. [Google Scholar]
- Garrido, S.; Santos, A.M.P.; dos Santos, A.; Ré, P. Spatial distribution and vertical migrations of fish larvae communities off Northwestern Iberia sampled with LHPR and Bongo nets. Estuar. Coast. Shelf Sci. 2009, 84, 463–475. [Google Scholar] [CrossRef]
- Sabatés, A. Distribution pattern of larval fish populations in the Northwestern Mediterranean. Mar. Ecol. Prog. Ser. 1990, 59, 75–82. [Google Scholar] [CrossRef]
- Bakun, A. Fronts and eddies as key structures in the habitat of marine fish larvae: Opportunity, adaptive response. Sci. Mar. 2006, 70, 105–122. [Google Scholar] [CrossRef]
- Secchi, E.R.; Danilewicz, D.; Ott, P.H.; Ramos, R.; Lazaro, M.; Marigo, J.; Wang, J.Y. Report of the Working Group on Stock Identity of Mackerel and Horse Mackerel. Lat. Am. J. Aquat. Mamm. 2002, 1, 47–54. [Google Scholar] [CrossRef]
- Garcia-Diaz, M.M.; Tuset, V.M.; González, J.A.; Socorro, J. Sex and reproductive aspects in Serranus cabrilla (Osteichthyes: Serranidae): Macroscopic and histological approaches. Mar. Biol. 1997, 127, 379–386. [Google Scholar] [CrossRef]
- ICESa. Report of the Workshop on Age Estimation of European Anchovy (Engraulis Encrasicolus). 2017, Volume 28. Available online: http://hdl.handle.net/10508/11100 (accessed on 6 November 2020).
- ICES. Report of the Benchmark Workshop on Pelagic Stocks, 6–10 February 2017, Lisbon, Portugal, 2017. p. 278. Available online: http://hdl.handle.net/10508/11076 (accessed on 6 November 2020).









| Family/Species | Code | % Oc | % RA | Family/Species | Code | % Oc | % RA |
|---|---|---|---|---|---|---|---|
| Family Gobiidae | G | 58.2 | 11.4 | Family Trachinidae | |||
| Family Carangidae | Trachinus draco | Td | 22.4 | 2.3 | |||
| Trachurus trachurus | Tt | 62.7 | 9.2 | Echiichthys vipera | Ev | 6 | 0.4 |
| Trachurus mediterraneus | Tm | 11.9 | 1.5 | Family Gadidae | |||
| Family Labridae | Gadiculus argenteus | Ga | 11.9 | 1.8 | |||
| Coris julis | Cj | 49.3 | 6.2 | Pollachius pollachius | Ppll | 1.5 | 0.3 |
| Ctenolabrus rupestris | Cr | 19.4 | 2.3 | Raniceps raninus | Rr | 3 | 0.2 |
| Symphodus melops | Sm | 7.5 | 0.5 | Family Scorpaenidae | |||
| Unidentified spp. | L | 1.5 | 0.2 | Scorpaena porcus | Spr | 26.9 | 2.2 |
| Labrus bergylta | Lb | 1.5 | 0.1 | Family Merluciidae | |||
| Family Serranidae | Merluccius merluccius | Mm | 19.4 | 2.1 | |||
| Serranus cabrilla | Scb | 76.1 | 8.5 | Family Caproidae | |||
| Serranus hepatus | Sh | 3 | 0.1 | Capros aper | Ca | 19.4 | 1.7 |
| Family Sparidae | Family Cepolidae | ||||||
| Pagellus acarne | Pa | 35.8 | 3.0 | Cepola macrophthalma | Cmc | 11.9 | 1.1 |
| Pagrus pagrus | Ppgr | 17.9 | 1.3 | Family Mugilidae | |||
| Boops boops | Bb | 9 | 1.0 | Mugil cephalus | Mc | 9 | 1.0 |
| Unidentified spp. | S | 9 | 1.1 | Family Mullidae | |||
| Diplodus spp. | Dspp | 9 | 0.5 | Mullus surmuletus | Ms | 10.4 | 1.0 |
| Pagellus bogaraveo | Pb | 3 | 0.4 | Family Triglidae | |||
| Pagellus erythrinus | Pe | 3 | 0.4 | Eutrigla gunardus | Eg | 4.5 | 0.5 |
| Family Sternoptychidae | Lepidotrigla cavillone | Lcv | 3 | 0.3 | |||
| Maurolicus muelleri | Mmll | 62.7 | 6.4 | Family Pleuronectidae | P | ||
| Argyropelecus hemigymnus | Ah | 6 | 0.3 | Unidentified spp. | 1.5 | 0.3 | |
| Family Blenniidae | Pleuronectes platessa | Ppl | 1.5 | 0.2 | |||
| Parablennius pilicornis | Pp | 40.3 | 4.3 | Family Scombridae | |||
| Parablennius tentacularis | Pt | 4.5 | 0.3 | Scomber colias | Sc | 3 | 0.4 |
| Lipophrys pholis | Lp | 1.5 | 0.2 | Family Scophthalmidae | Scph | 1.5 | 0.1 |
| Coryphoblennius galerita | Cg | 1.5 | 0.1 | Unidentified spp | |||
| Parablennius gattorugine | Pg | 1.5 | 0.1 | Zeugopterus punctatus | Zp | 1.5 | 0.2 |
| Family Bothidae | Family Soleidae | ||||||
| Arnoglossus thori | At | 35.8 | 3.7 | Pegusa lascaris | Pl | 1.5 | 0.2 |
| Arnoglossus laterna | Al | 10.4 | 1.0 | Microchirus variegatus | Mv | 1.5 | 0.1 |
| Arnoglossus imperialis | Ai | 1.5 | 0.1 | Family Gobiesocidae | |||
| Arnoglossus spp. | Aspp | 1.5 | 0.0 | Diplecogaster bimaculata | Db | 1.5 | 0.1 |
| Family Myctophidae | Lepadogaster candollei | Lcn | 1.5 | 0.03 | |||
| Ceratoscopelus maderensis | Cmd | 14.9 | 1.3 | Family Gonostomatidae | |||
| Lampanyctus crocodilus | Lc | 10.4 | 1.3 | Cyclothone braueri | Cb | 3 | 0.1 |
| Myctophum punctatum | Mp | 17.9 | 1.2 | Family Paralepididae | |||
| Benthosema glaciale | Bg | 13.4 | 0.7 | Lestidiops sphyrenoides | Ls | 1.5 | 0.1 |
| Unidentified spp. | M | 1.5 | 0.1 | Family Syngnathidae | |||
| Notoscopelus elongatus | Ne | 3 | 0.0 | Nerophis lumbriciformis | Nl | 1.5 | 0.1 |
| Family Callionymidae | Cspp | 40.3 | 4.3 | Family Lotidae | |||
| Unidentified individuals | U | 29.9 | 4.2 | Gaidropsarus vulgaris | Gpv | 1.5 | 0.1 |
| Family Clupeidae | Family Argentinidae | ||||||
| Sardina pilchardus | Sp | 28.4 | 3.2 | Argentina spyiraena | As | 1.5 | 0.04 |
| Family Engraulidae | |||||||
| Engraulis encrasicolus | Ee | 26.9 | 2.7 |
| Diff. | Lwr. | Upr. | p Adj. | |
|---|---|---|---|---|
| [0–20 m]–[20–40 m] | 0.07 | −0.03 | 0.18 | 0.300 |
| [0–20 m]–[40–60 m] | 0.14 | 0.02 | 0.25 | 0.008 |
| [0–20 m]–[60–100 m] | −0.01 | −0.13 | 0.10 | 0.996 |
| [0–20 m]–>100 m | 0.31 | 0.19 | 0.42 | 0.000 |
| [20–40 m]–[40–60 m] | 0.06 | −0.05 | 0.18 | 0.522 |
| [20–40 m]–[60–100 m] | −0.09 | −0.20 | 0.02 | 0.205 |
| [20–40 m] >100 m | 0.38 | 0.26 | 0.49 | 0.000 |
| [40–60 m]–[60–100 m] | −0.15 | −0.27 | −0.03 | 0.006 |
| [40–60 m]–>100 m | 0.44 | 0.32 | 0.57 | 0.000 |
| [60–100 m]–>100 m | 0.29 | 0.17 | 0.41 | 0.000 |
| 0–20 m | 20–40 m | 40–60 m | 60–100 m | >100 m | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Day | Night | Day | Night | Day | Night | Day | Night | Day | Night |
| A. thori | 55.0 ± 6.6 | 72.2 ± 12.2 | 88.4 ± 53.6 | 38.1 ± 6.6 | 38.4 ± 6.4 | 47.6 | 13.9 | 20.0 | ||
| C. aper | 70.1 ± 30.3 | 60.0 ± 40.0 | 94.0 ± 54.1 | 34.5 | 32.3 | 14.1 | ||||
| C. maderensis | 39.2 ± 13.5 | 160.1 ± 88.3 | 32.1 ± 6.4 | 84.8 ± 24.0 | 62.7 ± 28.2 | |||||
| C. julis | 184.6 ± 58.6 | 286.0 ± 98.3 | 127.5 ± 38.1 | 58.2 ± 5.6 | 84.2 ± 28.1 | 41.1 ± 6.6 | 21.9 ± 4.1 | 21.7 | 6.3 | |
| E. encrasicolus | 34.3 ± 8.7 | 76.1 ± 30.2 | 40.8 ± 6.6 | 45.2 ± 6.1 | 34.0 ± 1.7 | 53.4 ± 18.8 | 14.9 ± 1.0 | |||
| L. crocodilus | 43.5 | 73.8 ± 26.2 | 35.7 | 123.7 ± 59.1 | 90.6 ± 60.9 | |||||
| M. muelleri | 58.4 ± 13.0 | 223.9 ± 163.5 | 53.2 ± 5.6 | 98.7 ±56.4 | 39.2 ± 0.8 | 63.2 ± 13.7 | 24.4 ± 4.3 | 34.9 ± 5.7 | 40.2 ± 18.2 | 43.6 ± 24.1 |
| M. merlucius | 35.7 | 51.1 ± 13.3 | 26.0 ± 9.4 | 34.6 ± 2.4 | 13.5 ± 5.3 | 17.0 ± 2.0 | 33.3 | |||
| M. punctatum | 45.5 | 40.0 | 47.0 ± 15.4 | 94.7 ± 33.4 | 11.4 | 27.8 ± 10.1 | ||||
| P. acarne | 81.5 ± 16.9 | 52.8 ± 10.3 | 37.0 | 45.5 | 32.3 | 18.5 | ||||
| P. pilicornis | 72.5 ± 12.2 | 63.5 ± 8.9 | 44.7 ± 20.8 | 76.3 ± 7.3 | 32.3 | 34.5 | 20.4 | 20.0 | ||
| S. pilchardus | 108.8 ± 18.1 | 104.3 ± 24.5 | 90.1 ± 28.9 | 127 ± 79.0 | 44.4 ± 12.4 | 29.3 ± 3.0 | 46.8 ± 24.6 | 17.9 | ||
| S. cabrilla | 158.7 ± 26.8 | 251.7 ± 45.2 | 152.6 ± 37.4 | 86.4 ± 29.0 | 86.5 ± 19.8 | 18.2 | 22.8 ± 6.1 | 27.0 | ||
| T. draco | 55.2 ± 9.0 | 113.9 ± 43.5 | 29.4 ± 6.2 | 69.0 | 41.0 ± 4.7 | 13.0 | ||||
| T. trachurus | 134.1 ± 44.5 | 142.8 ± 44.8 | 254.1 ± 89.2 | 58.7 ± 12.1 | 79.3 ± 20.5 | 75.1 ± 21.2 | 16.2 ± 1.8 | 60.0 | 31.3 | |
| All fish larvae | 89.7 ± 1.0 | 127.1 ± 7.3 | 109.8 ± 14.0 | 97.8 ± 14.9 | 81.5 ± 18.5 | 65.9 ± 13.5 | 30.8 ± 8.4 | 30.2 ± 5.6 | 34.1 ± 3.6 | 48.6 ± 10.9 |
| Mesozooplankton | 1037.0 ± 106.3 | 1277.4 ± 148.1 | 975.1 ± 98.4 | 1136.0 ± 136.9 | 723.0 ± 78.0 | 719.5 ± 70.4 | 327.6 ± 29.2 | 347.0 ± 26.8 | 219.5 ± 25.9 | 236.8 ± 39.3 |
| Species | R |
|---|---|
| A. thori | 0.54 * |
| C. aper | 0.19 |
| C. maderensis | −0.3 |
| C. julis | 0.15 |
| E. encrasicolus | 0.32 |
| L. crocodilus | 0.19 |
| M. muelleri | 0.37 |
| M. merlucius | −0.08 |
| M. punctatum | −0.29 |
| P. acarne | −0.04 |
| S. pilchardus | 0.39 * |
| S. cabrilla | −0.01 |
| T. draco | 0.21 |
| T. trachurus | 0.35 |
| All fish larvae | 0.53 * |
| Species | DVM | T-Statistic | p-Value | Low C.I. | High C.I. |
|---|---|---|---|---|---|
| A. thori | 3.6 | 0.4 | 0.7 | −16.6 | 23.9 |
| C. aper | −9.5 | −0.6 | 0.6 | −60.6 | 41.6 |
| C. maderensis | −5.9 | −1.1 | 0.3 | −19.2 | 7.33 |
| C. julis | 11.4 | 3.3 | 0.0 ** | 4.33 | 18.5 |
| E. encrasicolus | 7.1 | 1.3 | 0.2 | −4.52 | 18.8 |
| M. muelleri | 27.1 | 2.7 | 0.0 * | 6.42 | 47.8 |
| M. punctatum | −1.9 | −0.2 | 0.9 | −24.8 | 21.1 |
| P. acarne | 2.1 | 0.4 | 0.7 | −8.76 | 13.0 |
| P. pilicornis | −4.8 | −0.8 | 0.4 | −17.9 | 8.29 |
| S. pilchardus | −4.7 | −0.4 | 0.7 | −31.9 | 22.6 |
| S. cabrilla | 9.5 | 2.3 | 0.0 * | 1.13 | 17.9 |
| T. draco | 13.3 | 1.9 | 0.1 | −2.34 | 28.9 |
| T. trachurus | 5.2 | 1.2 | 0.2 | −3.61 | 14.0 |
| All fish larvae | 3.9 | 0.6 | 0.5 | −8.4 | 16.2 |
| Mesozooplankton | 1.2 | 0.2 | 0.8 | −9.4 | 11.8 |
| Species | Mean Larval Length Per Depth Stratum | Two-Way ANOVA p-Values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 m | 21–40 m | 41–60 m | 61–100 m | >100 m | |||||||||
| D | N | D | N | D | N | D | N | D | N | D/N | Depth | Time x Depth | |
| A. thori | 3.7 ± 0.6 (8) | 4.7 ± 0.6 (10) | 4.9 ± 0.3 (16) | 4.4 ± 1.0 (4) | 5.4 ± 0.2 (5) | 5.1 (1) | 6.6 (1) | 4.5 (1) | ns | ns | ns | ||
| C. aper | 3.1 ± 0.3 (5) | 4 ± 0.3 (2) | 3.5 ± 0.5 (13) | 2.3 (1) | 4.1 (1) | ns | ns | ns | |||||
| C. maderensis | 3.6 ± 0.5 (3) | 5.7 ± 0.3 (14) | 4.4 ± 1.9 (3) | 3.8 ± 0.7 (9) | 3.6 (1) | 3.9 ± 0.4 (3) | ns | ns | ns | ||||
| C. julis | 2.7 ± 1 (40) | 2.9 ± 1.6 (85) | 3.9 ± 0.1 (61) | 2.8 ± 0.8 (4) | 3.9 ± 0.2 (14) | 4.4 ± 1.0 (2) | 4.5 ± 0.3 (3) | 4.1 (1) | 2.4 (1) | ns | <0.01 | ns | |
| E. encrasicolus | 5.2 ± 0.9 (4) | 7.1 ± 0.8 (10) | 6.7 ± 1.1 (6) | 6.1 ± 1.7 (3) | 8.3 ± 2.6 (2) | 7.2 ± 1.8 (4) | 8.6 ± 1.2 (2) | ns | ns | ns | |||
| L. crocodilus | 5.4 (1) | 5.4 ± 0.9 (5) | 5.6 (1) | 4.3 ± 0.4 (10) | 4.9 (1) | 2.6 ± 0.2 (9) | ns | <0.01 | ns | ||||
| M. muelleri | 7.2 ± 2.3 (2) | 9.3 ± 0.4 (13) | 4.2 ± 0.6 (2) | 6.5 ± 0.5 (12) | 4.5 ± 0.7 (3) | 6.9 ± 0.8 (13) | 5.9 ± 0.4 (16) | 7 ± 0.3 (37) | 7 ± 0.2 (53) | 7.3 ± 0.3 (34) | <0.05 | <0.01 | ns |
| M. merluccius | 6.5 ± 3.1 (3) | 11.5 (1) | 3.1 (1) | 3.4 (1) | - | - | - | ||||||
| M. punctatum | 3.6 (1) | 6.3 (1) | 6.5 ± 1.4 (4) | 4.7 ± 0.3 (12) | 9.4 (1) | 6.0 ± 0.6 (6) | ns | ns | ns | ||||
| P. acarne | 3.5 ± 1.9 (29) | 3.1 ± 2.8 (8) | 4.9 (1) | 2.4 (1) | 3.3 (1) | 3.8 (1) | ns | ns | ns | ||||
| P. pilicornis | 3.5 ± 0.3 (21) | 3.7 ± 0.3 (18) | 2.9 ± 0.5 (6) | 3.8 ± 0.3 (5) | 4.8 (1) | 3.7 ± 0.8 (2) | 2 (1) | 2.2 (1) | ns | ns | ns | ||
| S. pilchardus | 4.9 ± 0.8 (14) | 5.8 ± 0.9 (10) | 7.5 ± 0.7 (12) | 6.8 ± 1.0 (8) | 9.5 ± 0.4 (4) | 6.7 ± 3.9 (2) | 9.5 ± 1.4 (3) | ns | <0.01 | ns | |||
| S. cabrilla | 3.4 ± 0.9 (99) | 3.2 ± 1.3 (93) | 4 ± 1.3 (83) | 3.6 ± 0.4 (10) | 4.6 ± 0.2 (25) | 2.7 (1) | 4.7 ± 0.3 (12) | <0.01 | ns | ns | |||
| T. draco | 2.7 ± 1.0 (6) | 2.6 ± 0.4 (7) | 4.1 ± 1.4 (4) | 3.9 ± 2.3 (2) | 5.1 ± 0.0 (2) | 5.9 (1) | ns | ns | ns | ||||
| T. trachurus | 2.6 ± 0.2 (53) | 3 ± 0.2 (49) | 2.8 ± 0.1 (127) | 3.9 ± 0.4 (9) | 4.5 ± 0.2 (25) | 3.4 ± 0.3 (11) | 3.9 ± 0.8 (5) | 3.6 ± 1.0 (3) | 2.0 (1) | ns | <0.01 | <0.01 | |
| Diversity Index | Species Richness | Larval Fish Abundance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff | SE | Edf | p | Coeff | SE | Edf | p | Coeff | SE | Edf | p | |
| Intercept | −0.99 | 0.98 | 0.317 | −1.57 | 1.56 | <0.001 | −1.57 | 1.67 | 0.347 | |||
| SSS | 2.28 | <0.001 | 0.33 | 0.12 | 2.5 | <0.001 | ||||||
| SST | 0.11 | 0.05 | <0.1 | 0.17 | 0.31 | 0.10 | <0.01 | |||||
| LogDepth | −1.44 | 0.25 | <0.001 | |||||||||
| GV | 0.03 | 0.01 | <0.01 | 0.03 | 0.011 | <0.001 | 0.09 | 0.02 | <0.001 | |||
| Abdzoo | 5.36 | <0.001 | ||||||||||
| Deviance Explained | 59% | 51.5% | 66.1% | |||||||||
| Dispersion parameter | 0.21 | 1.12 | 1.01 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rábade Uberos, S.; Vergara Castaño, A.R.; Domínguez-Petit, R.; Saborido-Rey, F. Larval Fish Community in the Northwestern Iberian Upwelling System during the Summer Period. Oceans 2021, 2, 700-722. https://doi.org/10.3390/oceans2040040
Rábade Uberos S, Vergara Castaño AR, Domínguez-Petit R, Saborido-Rey F. Larval Fish Community in the Northwestern Iberian Upwelling System during the Summer Period. Oceans. 2021; 2(4):700-722. https://doi.org/10.3390/oceans2040040
Chicago/Turabian StyleRábade Uberos, Sonia, Alba Ruth Vergara Castaño, Rosario Domínguez-Petit, and Fran Saborido-Rey. 2021. "Larval Fish Community in the Northwestern Iberian Upwelling System during the Summer Period" Oceans 2, no. 4: 700-722. https://doi.org/10.3390/oceans2040040
APA StyleRábade Uberos, S., Vergara Castaño, A. R., Domínguez-Petit, R., & Saborido-Rey, F. (2021). Larval Fish Community in the Northwestern Iberian Upwelling System during the Summer Period. Oceans, 2(4), 700-722. https://doi.org/10.3390/oceans2040040