Effect of TiO2 Photoanodes Morphology and Dye Structure on Dye-Regeneration Kinetics Investigated by Scanning Electrochemical Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
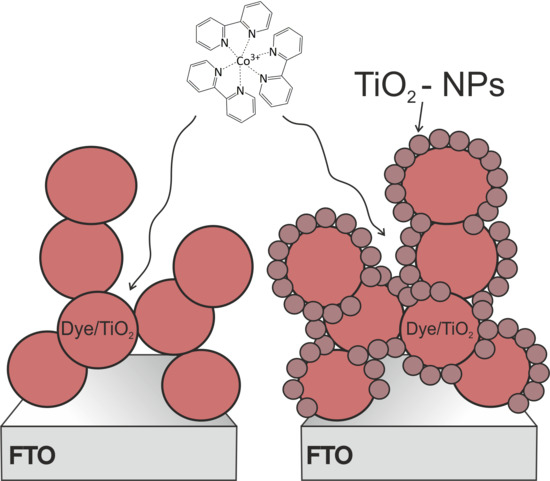
2.2. TiO2 Film Production
2.3. Sensitization of the Photoanodes
2.4. SECM Instrumentation
3. Result
3.1. SECM Approach Curves
3.1.1. Treatment of the Approach Curves
3.1.2. Effect of Post-TiCl4 Treatment
3.2. Transient Photocurrents
4. Discussion
4.1. Determination of Regeneration Rate Constant
4.2. Light-Harvesting Efficiency
4.3. Transient Photocurrent
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal titanium dioxide films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)-Based Redox Electrolyte Exceed 12% Efficiency [Erratum to document cited in CA156: 15579]. Science 2011, 334, 1203. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Snaith, H.J. Estimating the Maximum Attainable Efficiency in Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2010, 20, 13–19. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron mobility and injection dynamics in mesoporous ZnO, SnO2, and TiO2 films used in dye-sensitized solar cells. ACS Nano 2011, 5, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Peter, L. “Sticky Electrons” Transport and Interfacial Transfer of Electrons in the Dye-Sensitized Solar Cell. Acc. Chem. Res. 2009, 42, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Peter, L. Characterization and Modeling of Dye-Sensitized Solar Cells: A Toolbox Approach. In Dye-Sensitized Solar Cells; Kalyanasundaram, K., Ed.; EPFL Press: Lausanne, Switzerland, 2010; pp. 323–402. [Google Scholar]
- Hao, Y.; Yang, W.; Karlsson, M.; Cong, J.; Wang, S.; Li, X.; Xu, B.; Hua, J.; Kloo, L.; Boschloo, G. Efficient Dye-Sensitized Solar Cells with Voltages Exceeding 1 V through Exploring Tris(4-alkoxyphenyl)amine Mediators in Combination with the Tris(bipyridine) Cobalt Redox System. ACS Energy Lett. 2018, 3, 1929–1937. [Google Scholar] [CrossRef]
- Listorti, A.; O’Regan, B.; Durrant, J.R. Electron Transfer Dynamics in Dye-Sensitized Solar Cells. Chem. Mater. 2011, 23, 3381–3399. [Google Scholar] [CrossRef]
- Chandiran, A.K.; Abdi-Jalebi, M.; Nazeeruddin, M.K.; Grätzel, M. Analysis of electron transfer properties of ZnO and TiO2 photoanodes for dye-sensitized solar cells. ACS Nano 2014, 8, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Nĕmec, H.; Rochford, J.; Taratula, O.; Galoppini, E.; Kuzel, P.; Polívka, T.; Yartsev, A.; Sundström, V. Influence of the electron-cation interaction on electron mobility in dye-sensitized ZnO and TiO2 nanocrystals: A study using ultrafast terahertz spectroscopy. Phys. Rev. Lett. 2010, 104, 197401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, M.; Edvinsson, T.; Hagfeldt, A.; Boschloo, G. Comparison of dye-sensitized ZnO and TiO2 solar cells: Studies of charge transport and carrier lifetime. J. Phys. Chem. C 2007, 111, 1035–1041. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates Jr, J.T. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Grätzel, M. Conversion of light to electricity by cis-X2bis(2,2’-bipyridyl-4,4’-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline TiO2 electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Park, N.-G.; Schlichthörl, G.; van de Lagemaat, J.; Cheong, H.M.; Mascarenhas, A.; Frank, A.J. Dye-Sensitized TiO2 Solar Cells: Structural and Photoelectrochemical Characterization of Nanocrystalline Electrodes Formed from the Hydrolysis of TiCl4. J. Phys. Chem. B 1999, 103, 3308–3314. [Google Scholar] [CrossRef]
- Sommeling, P.M.; O’Regan, B.C.; Haswell, R.R.; Smit, H.J.P.; Bakker, N.J.; Smits, J.J.T.; Kroon, J.M.; van Roosmalen, J.A.M. Influence of a TiCl4 post-treatment on nanocrystalline TiO2 films in dye-sensitized solar cells. J. Phys. Chem. B 2006, 110, 19191–19197. [Google Scholar] [CrossRef]
- Barbé, C.J.; Arendse, F.; Comte, P.; Jirousek, M.; Lenzmann, F.; Shklover, V.; Grätzel, M. Nanocrystalline Titanium Oxide Electrodes for Photovoltaic Applications. J. Am. Ceram. Soc. 1997, 80, 3157–3171. [Google Scholar] [CrossRef]
- Ito, S.; Liska, P.; Comte, P.; Charvet, R.; Péchy, P.; Bach, U.; Schmidt-Mende, L.; Zakeeruddin, S.M.; Kay, A.; Nazeeruddin, M.K.; et al. Control of dark current in photoelectrochemical (TiO2/I−-I3−)) and dye-sensitized solar cells. Chem. Commun. 2005, 4351–4353. [Google Scholar] [CrossRef] [Green Version]
- O’Regan, B.C.; Durrant, J.R.; Sommeling, P.M.; Bakker, N.J. Influence of the TiCl4 Treatment on Nanocrystalline TiO2 Films in Dye-Sensitized Solar Cells. 2. Charge Density, Band Edge Shifts, and Quantification of Recombination Losses at Short Circuit. J. Phys. Chem. C 2007, 111, 14001–14010. [Google Scholar] [CrossRef]
- Zeng, L.-Y.; Dai, S.-Y.; Wang, K.-J.; Pan, X.; Shi, C.-W.; Guo, L. Mechanism of Enhanced Performance of Dye-Sensitized Solar Cell Based TiO2 Films Treated by Titanium Tetrachloride. Chin. Phys. Lett. 2004, 21, 1835–1837. [Google Scholar]
- Yella, A.; Mathew, S.; Aghazada, S.; Comte, P.; Grätzel, M.; Nazeeruddin, M.K. Dye-sensitized solar cells using cobalt electrolytes: The influence of porosity and pore size to achieve high-efficiency. J. Mater. Chem. C 2017, 5, 2833–2843. [Google Scholar] [CrossRef]
- Zukalová, M.; Zukal, A.; Kavan, L.; Nazeeruddin, M.K.; Liska, P.; Grätzel, M. Organized mesoporous TiO2 films exhibiting greatly enhanced performance in dye-sensitized solar cells. Nano Lett. 2005, 5, 1789–1792. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Zakeeruddin, S.M.; Humphry-Baker, R.; Liska, P.; Charvet, R.; Comte, P.; Nazeeruddin, M.K.; Pechy, P.; Takata, M.; Miura, H.; et al. High-efficiency organic-dye-sensitized solar cells controlled by nanocrystalline-TiO2 electrode thickness. Adv. Mater. 2006, 18, 1202–1205. [Google Scholar] [CrossRef]
- Nusbaumer, H.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M. An Alternative Efficient Redox Couple for the Dye-Sensitized Solar Cell System. Chem. Eur. J. 2003, 9, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hao, Y.; Cabot, A.; Johansson, E.M.J.; Boschloo, G.; Hagfeldt, A. Cobalt(II/III) redox electrolyte in ZnO nanowire-based dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 1902–1906. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.; Wang, P.; Ito, S.; Zakeeruddin, S.M.; Grätzel, M. Stable Mesoscopic Dye-Sensitized Solar Cells Based on Tetracyanoborate Ionic Liquid Electrolyte. J. Am. Chem. Soc. 2006, 128, 7732–7733. [Google Scholar] [CrossRef]
- Tsao, H.N.; Comte, P.; Yi, C.; Grätzel, M. Avoiding Diffusion Limitations in Cobalt(III/II)-Tris (2, 2′-Bipyridine)-Based Dye-Sensitized Solar Cells by Tuning the Mesoporous TiO2 Film Properties. ChemPhysChem 2012, 13, 2976–2981. [Google Scholar] [CrossRef]
- Tefashe, U.M.; Rudolph, M.; Miura, H.; Schlettwein, D.; Wittstock, G. Photovoltaic characteristics and dye regeneration kinetics in D149-sensitized ZnO with varied dye loading and film thickness. Phys. Chem. Chem. Phys. 2012, 14, 7533–7542. [Google Scholar] [CrossRef]
- Rahman, M.; Wei, M.; Xie, F.; Khan, M. Efficient Dye-Sensitized Solar Cells Composed of Nanostructural ZnO Doped with Ti. Catalysts 2019, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Nonomura, K.; Schlettwein, D.; Zhao, C.; Wittstock, G. Photoelectrochemical Kinetics of Eosin Y-Sensitized Zinc Oxide Films Investigated by Scanning Electrochemical Microscopy. Chem. Eur. J. 2006, 12, 5832–5839. [Google Scholar] [CrossRef]
- Ellis, H.; Schmidt, I.; Hagfeldt, A.; Wittstock, G.; Boschloo, G. Influence of Dye Architecture of Triphenylamine Based Organic Dyes on the Kinetics in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2015, 119, 21775–21783. [Google Scholar] [CrossRef]
- Falgenhauer, J.; Richter, C.; Miura, H.; Schlettwein, D. Stable Sensitization of ZnO by Improved Anchoring of Indoline Dyes. ChemPhysChem 2012, 13, 2893–2897. [Google Scholar] [CrossRef] [PubMed]
- Ruess, R.; Scarabino, S.; Ringleb, A.; Nonomura, K.; Vlachopoulos, N.; Hagfeldt, A.; Wittstock, G.; Schlettwein, D. Diverging Surface Reactions at TiO2- or ZnO-Based Photoanodes in Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2019, 21, 13047–13057. [Google Scholar] [CrossRef]
- Kozma, E.; Concina, I.; Braga, A.; Borgese, L.; Depero, L.E.; Vomiero, A.; Sberveglieri, G.; Catellani, M. Metal-free organic sensitizers with a sterically hindered thiophene unit for efficient dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 13785. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, N.; Wang, Y.; Li, R.; Yuan, Y.; Wang, P. Modulating the assembly of organic dye molecules on titania nanocrystals via alkyl chain elongation for efficient mesoscopic cobalt solar cells. Phys. Chem. Chem. Phys. 2012, 14, 8282–8286. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.W.; Kim, Y.S. Theoretical study of indoline dyes for dye-sensitized solar cells. Thin Solid Films 2010, 518, 6558–6563. [Google Scholar] [CrossRef]
- Murakami, T.N.; Koumura, N.; Kimura, M.; Mori, S. Structural effect of donor in organic dye on recombination in dye-sensitized solar cells with cobalt complex electrolyte. Langmuir 2014, 30, 2274–2279. [Google Scholar] [CrossRef]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Jiang, R.; Canto-Aguilar, E.J.; Oskam, G.; Boschloo, G. Improving the mass transport of copper-complex redox mediators in dye-sensitized solar cells by reducing the inter-electrode distance. Phys. Chem. Chem. Phys. 2017, 19, 32132–32142. [Google Scholar] [CrossRef]
- Heiniger, L.-P.; Giordano, F.; Moehl, T.; Grätzel, M. Mesoporous TiO2 Beads Offer Improved Mass Transport for Cobalt-Based Redox Couples Leading to High Efficiency Dye-Sensitized Solar Cells. Adv. Energy Mater. 2014, 4, 1400168. [Google Scholar] [CrossRef]
- Tefashe, U.M.; Nonomura, K.; Vlachopoulos, N.; Hagfeldt, A.; Wittstock, G. Effect of Cation on Dye Regeneration Kinetics of N719-Sensitized TiO2 Films in Acetonitrile-Based and Ionic-Liquid-Based Electrolytes Investigated by Scanning Electrochemical Microscopy. J. Phys. Chem. C 2012, 116, 4316–4323. [Google Scholar] [CrossRef]
- Nunes Kirchner, C.; Hallmeier, K.H.; Szargan, R.; Raschke, T.; Radehaus, C.; Wittstock, G. Evaluation of Thin Film Titanium Nitride Electrodes for Electroanalytical Applications. Electroanalysis 2007, 19, 1023–1031. [Google Scholar] [CrossRef]
- Feldt, S.M.; Lohse, P.W.; Kessler, F.; Nazeeruddin, M.K.; Grätzel, M.; Boschloo, G.; Hagfeldt, A. Regeneration and recombination kinetics in cobalt polypyridine based dye-sensitized solar cells, explained using Marcus theory. Phys. Chem. Chem. Phys. 2013, 15, 7087–7097. [Google Scholar] [CrossRef] [PubMed]
- Cornut, R.; Lefrou, C. New analytical approximation of feedback approach curves with a microdisk SECM tip and irreversible kinetic reaction at the substrate. J. Electroanal. Chem. 2008, 621, 178–184. [Google Scholar] [CrossRef]
- Tefashe, U.M.; Loewenstein, T.; Miura, H.; Schlettwein, D.; Wittstock, G. Scanning electrochemical microscope studies of dye regeneration in indoline (D149)-sensitized ZnO photoelectrochemical cells. J. Electroanal. Chem. 2010, 650, 24–30. [Google Scholar] [CrossRef]
- Cao, F.; Oskam, G.; Searson, P.C. Electron Transport in Porous Nanocrystalline TiO2 Photoelectrochemical Cells. J. Phys. Chem. 1996, 100, 17021–17027. [Google Scholar] [CrossRef]
- Walker, A.B.; Peter, L.M.; Martínez, D.; Lobato, K. Transient Photocurrents in Dye-Sensitized Nanocrystalline Solar Cells. Chimia 2007, 61, 792–795. [Google Scholar] [CrossRef]
- Nelson, J.J.; Amick, T.J.; Elliott, C.M. Mass Transport of Polypyridyl Cobalt Complexes in Dye-Sensitized Solar Cells with Mesoporous TiO2 Photoanodes. J. Phys. Chem. C 2008, 112, 18255–18263. [Google Scholar] [CrossRef]
- Guillen, E.; Peter, L.M.; Anta, J.A. Electron Transport and Recombination in ZnO-Based Dye-Sensitized Solar Cells. J. Phys. Chem. C 2011, 115, 22622–22632. [Google Scholar] [CrossRef]
- Keis, K.; Vayssieres, L.; Rensmo, H.; Lindquist, S.-E.; Hagfeldt, A. Photoelectrochemical Properties of Nano- to Microstructured ZnO Electrodes. J. Electrochem. Soc. 2001, 148, A149–A155. [Google Scholar] [CrossRef]
- Tachibana, Y.; Hara, K.; Sayama, K.; Arakawa, H. Quantitative Analysis of Light-Harvesting Efficiency and Electron-Transfer Yield in Ruthenium-Dye-Sensitized Nanocrystalline TiO2 Solar Cells. Chem. Mater. 2002, 14, 2527–2535. [Google Scholar] [CrossRef]
- Bisquert, J.; Vikhrenko, V.S. Interpretation of the Time Constants Measured by Kinetic Techniques in Nanostructured Semiconductor Electrodes and Dye-Sensitized Solar Cells. J. Phys. Chem. B 2004, 108, 2313–2322. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarabino, S.; Nonomura, K.; Vlachopoulos, N.; Hagfeldt, A.; Wittstock, G. Effect of TiO2 Photoanodes Morphology and Dye Structure on Dye-Regeneration Kinetics Investigated by Scanning Electrochemical Microscopy. Electrochem 2020, 1, 329-343. https://doi.org/10.3390/electrochem1030021
Scarabino S, Nonomura K, Vlachopoulos N, Hagfeldt A, Wittstock G. Effect of TiO2 Photoanodes Morphology and Dye Structure on Dye-Regeneration Kinetics Investigated by Scanning Electrochemical Microscopy. Electrochem. 2020; 1(3):329-343. https://doi.org/10.3390/electrochem1030021
Chicago/Turabian StyleScarabino, Sabina, Kazuteru Nonomura, Nick Vlachopoulos, Anders Hagfeldt, and Gunther Wittstock. 2020. "Effect of TiO2 Photoanodes Morphology and Dye Structure on Dye-Regeneration Kinetics Investigated by Scanning Electrochemical Microscopy" Electrochem 1, no. 3: 329-343. https://doi.org/10.3390/electrochem1030021
APA StyleScarabino, S., Nonomura, K., Vlachopoulos, N., Hagfeldt, A., & Wittstock, G. (2020). Effect of TiO2 Photoanodes Morphology and Dye Structure on Dye-Regeneration Kinetics Investigated by Scanning Electrochemical Microscopy. Electrochem, 1(3), 329-343. https://doi.org/10.3390/electrochem1030021