Direct Alcohol Biomarkers Prediction Capacity on Relapse and Mortality in Liver Transplantation Candidates: A Follow-Up Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Subjects
2.2. Procedure and Outcome Selection
2.3. Statistical Analysis
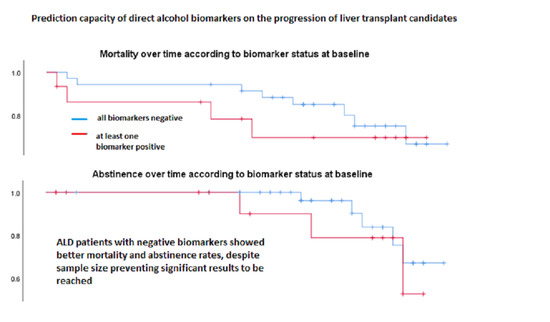
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beresford, T.P.; Lucey, M.R. Towards standardizing the alcoholism evaluation of potential liver transplant recipients. Alcohol Alcohol. 2018, 53, 135–144. [Google Scholar] [CrossRef]
- Telles-Correia, D.; Mega, I. Candidates for liver transplantation with alcoholic liver disease: Psychosocial aspects. World J. Gastroenterol. 2015, 21, 11027–11033. [Google Scholar] [CrossRef]
- DiMartini, A.F.; Dew, M.A. Monitoring alcohol use on the liver transplant wait list: Therapeutic and practical issues. Liver Transplant. 2012, 18, 1267–1269. [Google Scholar] [CrossRef]
- Staufer, K.; Yegles, M. Biomarkers for detection of alcohol consumption in liver transplantation. World J. Gastroenterol. 2016, 22, 3725–3734. [Google Scholar] [CrossRef]
- Kodali, S.; Kaif, M.; Tariq, R.; Singal, A.K. Alcohol relapse after liver transplantation for alcoholic cirrhosis-impact on liver graft and patient survival: A meta-analysis. Alcohol Alcohol. 2018, 53, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Rice, J.P.; Eickhoff, J.; Agni, R.; Ghufran, A.; Brahmbhatt, R.; Lucey, M.R. Abusive drinking after liver transplantation is associated with allograft loss and advanced allograft fibrosis. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2013, 19, 1377–1386. [Google Scholar] [CrossRef]
- Shawcross, D.L.; O’Grady, J.G. The 6-month abstinence rule in liver transplantation. Lancet 2010, 376, 216–217. [Google Scholar] [CrossRef]
- Allen, J.P.; Wurst, F.M.; Thon, N.; Litten, R.Z. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transplant. 2013, 19, 369–376. [Google Scholar] [CrossRef]
- Barrio, P.; Wurst, F.M.; Gual, A. New alcohol biomarkers. New challenges. Alcohol Alcohol. 2018, 53, 762–763. [Google Scholar] [CrossRef] [Green Version]
- Wurst, F.M.; Thon, N.; Yegles, M.; Schrück, A.; Preuss, U.W.; Weinmann, W. Ethanol metabolites: Their role in the assessment of alcohol intake. Alcohol. Clin. Exp. Res. 2015, 39, 2060–2072. [Google Scholar] [CrossRef] [Green Version]
- Dumortier, J.; Dharancy, S.; Cannesson, A.; Lassailly, G.; Rolland, B.; Pruvot, F.R.; Boillot, O.; Faure, S.; Guillaud, O.; Rigole-Donnadieu, H.; et al. Recurrent alcoholic cirrhosis in severe alcoholic relapse after liver transplantation: A frequent and serious complication. Am. J. Gastroenterol. 2015, 110, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Erard-Poinsot, D.; Guillaud, O.; Hervieu, V.; Thimonier, E.; Vallin, M.; Chambon-Augoyard, C.; Boillot, O.; Scoazec, J.Y.; Dumortier, J. Severe alcoholic relapse after liver transplantation: What consequences on the graft? A study based on liver biopsies analysis. Liver Transplant. 2016, 22, 773–784. [Google Scholar] [CrossRef]
- Chuncharunee, L.; Yamashiki, N.; Thakkinstian, A.; Sobhonslidsuk, A. Alcohol relapse and its predictors after liver transplantation for alcoholic liver disease: A systematic review and meta-analysis. BMC Gastroenterol. 2019, 19, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutsch-Link, S.; Weinrieb, R.M.; Jones, L.S.; Solga, S.F.; Weinberg, E.M.; Serper, M. Prior relapse, ongoing alcohol consumption, and failure to engage in treatment predict alcohol relapse after liver transplantation. Dig. Dis. Sci. 2020, 65, 2089–2103. [Google Scholar] [CrossRef] [PubMed]
- Lindenger, C.; Castedal, M.; Schult, A.; Aberg, F. Long-term survival and predictors of relapse and survival after liver transplantation for alcoholic liver disease. Scand. J. Gastroenterol. 2018, 53, 1553–1561. [Google Scholar] [CrossRef]
- Lombardo-Quezada, J.; Colmenero, J.; López-Pelayo, H.; Gavotti, C.; Lopez, A.; Crespo, G.; Lopez, E.; Gual, A.; Lligoña, A.; Navasa, M. Prediction of alcohol relapse among liver transplant candidates with less than 6 months of abstinence using the high-risk alcoholism relapse score. Liver Transplant. 2019, 25, 1142–1154. [Google Scholar] [CrossRef]
- Pose, E.; Torrents, A.; Reverter, E.; Perez-Campuzano, V.; Campos-Varela, I.; Avitabile, E.; Gratacós-Ginès, J.; Castellote, J.; Castells, L.; Colmenero, J.; et al. A notable proportion of liver transplant candidates with alcohol-related cirrhosis can be delisted because of clinical improvement. J. Hepatol. 2021. [Google Scholar] [CrossRef]
- Satapathy, S.K.; Thornburgh, C.; Heda, R.; Jiang, Y.; Kedia, S.K.; Nair, S.P.; Eason, J.D.; Maluf, D. Predicting harmful alcohol relapse after liver transplant: The HALT score. Clin. Transplant. 2020, 34. [Google Scholar] [CrossRef]
- Barrio, P.; Mondon, S.; Teixidor, L.; Ortega, L.; Vieta, E.; Gual, A. One year clinical correlates of EtGpositive urine screening in alcohol-dependent patients: A survival analysis. Alcohol Alcohol. 2017, 52, 460–465. [Google Scholar] [CrossRef]
- Barrio, P.; Gual, A.; Lligoña, A.; Teixidor, L.; Weinmann, W.; Yegles, M.; Wurst, F.M. Phosphatidylethanol for monitoring alcohol use in liver transplant candidates: An observational study. J. Clin. Med. 2020, 9, 3060. [Google Scholar] [CrossRef]
- Bohn, M.J.; Babor, T.F.; Kranzler, H.R. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. J. Stud. Alcohol 1995, 56, 423–432. Available online: http://www.ncbi.nlm.nih.gov/pubmed/7674678 (accessed on 31 May 2021). [CrossRef] [PubMed]
- Sobell, L.C.; Sobell, M.B. Timeline follow-back. In Measuring Alcohol Consumption; Litten, R.Z., Allen, J.P., Eds.; Humana Press: Totowa, NJ, USA, 1992; pp. 41–72. [Google Scholar] [CrossRef]
- Björnsson, E.; Olsson, J.; Rydell, A.; Fredriksson, K.; Eriksson, C.; Sjöberg, C.; Olausson, M.; Bäckman, L.; Castedal, M.; Friman, S. Long-term follow-up of patients with alcoholic liver disease after liver transplantation in Sweden: Impact of structured management on recidivism. Scand. J. Gastroenterol. 2005, 40, 206–216. [Google Scholar] [CrossRef]
- Kollmann, D.; Rasoul-Rockenschaub, S.; Steiner, I.; Freundorfer, E.; Györi, G.P.; Silberhumer, G.; Soliman, T.; Berlakovich, G.A. Good outcome after liver transplantation for ALD without a 6 months abstinence rule prior to transplantation including post-transplant CDT monitoring for alcohol relapse assessment—A retrospective study. Transpl. Int. 2016, 29, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Skladany, L.; Selcanova, S.A.; Koller, T. Alcohol use relapse following liver transplantation for alcoholic liver disease. Ann. Transplant. 2019, 24, 359–366. [Google Scholar] [CrossRef]
- Vassallo, G.A.; Tarli, C.; Rando, M.M.; Mosoni, C.; Mirijello, A.; Agyei-Nkansah, A.; Antonelli, M.; Sestito, L.; Perotti, G.; Di Giuda, D.; et al. Liver transplantation in patients with alcoholic liver disease: A retrospective study. Alcohol Alcohol. 2018, 53, 151–156. [Google Scholar] [CrossRef]
- Marroni, C.A.; de Medeiros Fleck, A., Jr.; Fernandes, S.A.; Galant, L.H.; Mucenic, M.; de Mattos Meine, M.H.; Mariante-Neto, G.; de Mello Brandão, A.B. Liver transplantation and alcoholic liver disease: History, controversies, and considerations. World J. Gastroenterol. 2018, 24, 2785–2805. [Google Scholar] [CrossRef]
- Mathurin, P.; Hadengue, A.; Bataller, R.; Addolorato, G.; Burra, P.; Burt, A.; Caballeria, J.; Cortez-Pinto, H.; Day, C.P.; Forrest, E.H.; et al. EASL clinical practical guidelines: Management of alcoholic liver disease. J. Hepatol. 2012, 57, 399–420. [Google Scholar] [CrossRef]
- Barrio, P.; Teixidor, L.; Rico, N.; Bruguera, P.; Ortega, L.; Bedini, J.L.; Gual, A. Urine ethyl glucuronide unraveling the reality of abstinence monitoring in a routine outpatient setting: A cross-sectional comparison with ethanol, self report and clinical judgment. Eur. Addict. Res. 2016, 22, 243–248. [Google Scholar] [CrossRef]
- Skipper, G.E.; Weinmann, W.; Thierauf, A.; Schaefer, P.; Wiesbeck, G.; Allen, J.P.; Miller, M.; Wurst, F.M. Ethyl glucuronide: A biomarker to identify alcohol use by health professionals recovering from substance use disorders. Alcohol Alcohol. 2004, 39, 445–449. [Google Scholar] [CrossRef]
- Wetterling, T.; Dibbelt, L.; Wetterling, G.; Göder, R.; Wurst, F.; Margraf, M.; Junghanns, K. Ethyl glucuronide (EtG): Better than breathalyser or self-reports to detect covert short-term relapses into drinking. Alcohol Alcohol. 2014, 49, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Arnts, J.; Vanlerberghe, B.T.K.; Roozen, S.; Crunelle, C.L.; Masclee, A.A.M.; Olde-Damink, S.W.M.; Heeren, R.M.A.; van Nuijs, A.; Neels, H.; Nevens, F.; et al. Diagnostic accuracy of biomarkers of alcohol use in patients with liver disease: A systematic review. Alcohol. Clin. Exp. Res. 2021, 45, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.H.; Koch, D.G.; Burgess, D.M.; Willner, I.R.; Reuben, A. Sensitivity and specificity of urinary ethyl glucuronide and ethyl sulfate in liver disease patients. Alcohol. Clin. Exp. Res. 2013, 37, 150–155. [Google Scholar] [CrossRef]
- Fleming, M.F.; Smith, M.J.; Oslakovic, E.; Lucey, M.R.; Vue, J.X.; Al-Saden, P.; Levitsky, J. Phosphatidylethanoldetects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol. Clin. Exp. Res. 2017, 41, 857–862. [Google Scholar] [CrossRef]
- Addolorato, G.; Vassallo, G.A.; Mirijello, A.; Gasbarrini, A. Diagnosis and management of alcohol use disorder in patients with liver disease: Lights and shadows. Neurotherapeutics 2020, 17, 127–141. [Google Scholar] [CrossRef]
- Andresen-Streichert, H.; Beres, Y.; Weinmann, W.; Schröck, A.; Müller, A.; Skopp, G.; Pischke, S.; Vettorazzi, E.; Lohse, A.; Nashan, B.; et al. Improved detection of alcohol consumption using the novel marker phosphatidylethanol in the transplant setting: Results of a prospective study. Transpl. Int. 2017, 30, 611–620. [Google Scholar] [CrossRef] [Green Version]


| Follow-Up Time (Months) | Cause of Death |
|---|---|
| 1 | Sudden death |
| 2 | Septic shock |
| 2 | Surgical complications |
| 3 | Unknown |
| 16 | Hepatic encephalpopathy and upper digestive tractbleeding |
| 19 | Cirrhosis |
| 20 | Sepsis |
| 21 | Hepatic encephalopathy |
| 24 | Hepatocarcinocma |
| 29 | Hepatic encephalopathy |
| 30 | Hepatic encephalopathy |
| 35 | Endocarditis |
| Covariate | Model 1 (Outcome: Mortality) | Model 2 (Outcome: Clinical Relapse) |
|---|---|---|
| Age | B = −0.2; p = 0.562 | B = −0.27; p = 0.016 |
| Sex | OR = 3.5; 95% CI 0.28–45 | OR = 26.7; p = 0.94 |
| All biomarkers negative | B = −0.6; p = 0.377 | B = −1.06; p = 0.22 |
| Ever-smoker | OR = 1.8; 95% CI 0.49–6.6 | OR = 0.9; 95% CI 0.15–5.5 |
| Duration of reported abstinence | B = 0.007; p = 0.43 | B = −0.02; p = 0.21 |
| Lifetime drug use | OR = 0.6; 95% CI 0.08–5.15 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrio, P.; Marco, O.; Druetta, M.; Tardon, L.; Lligonya, A.; Wurst, F.M.; Weinmann, W.; Yegles, M.; Gual, A. Direct Alcohol Biomarkers Prediction Capacity on Relapse and Mortality in Liver Transplantation Candidates: A Follow-Up Study. Transplantology 2021, 2, 246-252. https://doi.org/10.3390/transplantology2030023
Barrio P, Marco O, Druetta M, Tardon L, Lligonya A, Wurst FM, Weinmann W, Yegles M, Gual A. Direct Alcohol Biomarkers Prediction Capacity on Relapse and Mortality in Liver Transplantation Candidates: A Follow-Up Study. Transplantology. 2021; 2(3):246-252. https://doi.org/10.3390/transplantology2030023
Chicago/Turabian StyleBarrio, Pablo, Oriol Marco, Mauro Druetta, Laia Tardon, Anna Lligonya, Friedrich Martin Wurst, Wolfgang Weinmann, Michel Yegles, and Antoni Gual. 2021. "Direct Alcohol Biomarkers Prediction Capacity on Relapse and Mortality in Liver Transplantation Candidates: A Follow-Up Study" Transplantology 2, no. 3: 246-252. https://doi.org/10.3390/transplantology2030023
APA StyleBarrio, P., Marco, O., Druetta, M., Tardon, L., Lligonya, A., Wurst, F. M., Weinmann, W., Yegles, M., & Gual, A. (2021). Direct Alcohol Biomarkers Prediction Capacity on Relapse and Mortality in Liver Transplantation Candidates: A Follow-Up Study. Transplantology, 2(3), 246-252. https://doi.org/10.3390/transplantology2030023