1. Introduction
In the last decades, there has been an alarming increase in energy consumption worldwide. Within only the 20 years from 1995 to 2015 the increase exceeded 50%, from 8588.9 million tons oil equivalent (Mtoe) in 1995 to 13,147.3 Mtoe [
1] in 2015. Energy-intensive industries (EIIs) are one of the top energy consumers with a global share of 24%. Of this, up to 80% is met with fossil fuels and their associated energy systems. Current energy systems rely in general on burning fossil fuels, which are not renewable; they are distributed worldwide and are critically unsustainable to deliver [
2]. Most of the CO
2 emissions that cause global warming derive from solid fuel combustion [
3]. In 2016, 32.3 Gtn of CO
2 emissions resulted from solid fuel combustion. The industrial sector is responsible for 19% of these emissions [
4,
5]. A study from the International Energy Agency (IEA) [
6] showed that fossil fuels that can cause environmental issues when combusted, such as air pollution and climate change, still play a major role in energy sources globally. CO
2 emissions increased from 20.9 Gtn in 1990 to 28.8 Gtn in 2007 and are expected to rise to 40.2 Gtn in 2030, with an average yearly increase rate of 1.5% over the projection period [
5].
It is believed that replacing fossil fuels with biomass fractions in the fuel feed for combustion will lower the overall unwanted major emissions from power facilities as biomass fuels present lower amounts of some elements, such as sulfur. The European Union (EU) has started a progressive decarbonization with the aim to become carbon neutral by 2050. EIIs are expected to play an important role in this transition. Biomass will continue to have an important role in the EU energy mix as it is important among renewable sources, covering approximately 5% of the primary energy supply of the EU-27 [
4]. Emissions of complete combustion in biomass applications include CO
2, NO
x, N
2O, SO
x, HCl, and heavy metals, while emissions of incomplete combustion include CO and unwanted organic compounds, among others; particle emissions can be the result of both complete and incomplete combustion [
7]. Combustion of most biomass materials is known to result in lower emissions of SO
x, and NO
x, as biomass sulfur and nitrogen contents are low compared to fossil fuels; alkali-based compounds also have a retention effect on sulfur, resulting in an additional incremental reduction [
8]. On the other hand, co-firing of different biomass fuels and fossil fuel usually does not lead to reduced sulfur emissions because inherently existing potassium chloride has a higher reactivity with aluminum silicates than sulfur compounds [
9]. In most biomass materials, a significant amount of submicron fumes and vapor material can be formed in the flame that can pose a challenge to particulate emissions abatement equipment. This may lead to lower collection efficiencies and increased particulate emissions from the stack, which is likely a highly site-specific occurrence of interest in retrofit projects.
A very interesting study by Monika Zajemska et al. [
10] presents the emissions of gaseous pollutants from the co-firing of sunflower husk pellets according to metrological processes but also through a simulation program. The calculated concentration of sulfur dioxide in the flue gas was higher by about 200 ppm from measured concentration (355 ppm) and reached a value of 588 ppm. Higher levels were also observed for nitric oxide although not as large as in the case of SO
2; namely, the calculated concentration reached a value of 192 ppm, and the measured concentration was equal to 162 ppm.
It is noted that there is renewed interest in many industrial countries in biomass combustion as a result of environmental and climate change concerns and because of energy security supplies in a world where fossil fuels are concentrated in a few countries and resources are finite. In addition, biomass combustion leads to reduction of net carbon dioxide (CO
2) and to better waste management, mainly due to the CO
2 neutrality and large availability of biomass [
11]. Biomass combustion or co-combustion with fossil fuels can significantly reduce CO
2 emissions from energy production. It is asserted that although biomass has the advantage of CO
2 neutrality, or nearly so, there are potential problems concerning the environment, such as NO
x and CO emissions, noted as the most considerable gaseous pollutants during biomass combustion [
12,
13].
The several economic and environmental advantages of biomass combustion are offset by its major disadvantage, which is its low energy potential, especially compared to fossil fuels [
14]. It is therefore quite difficult to meet the large amounts of energy required, mainly in industry, by biomass combustion. This is the main reason why biomass/fossil fuel co-firing technology has been developed and largely implemented worldwide. The co-firing of biomass with fossil fuels is a flexible and easily applicable treatment. No specialized burners are required as the biomass can be burned in all types of kilns without creating technical problems, while, depending on the energy requirements, the percentage of fossil fuels that the biomass replaces may vary. The technology of co-firing has been tested in various sectors for several years, but a great growth has been presented in the field of electricity generation, where it is now an extremely efficient process. At the beginning of the second decade of the 21st century, more than 220 power plants were put into operation with biomass co-firing technology. The majority of these power plants are located in Europe and mainly in the Nordic countries, where in Finland alone there are more than 70 units and in Sweden 15 units [
15].
In the United Kingdom, co-firing was not commercially productive until 2002. Producing 286 GWh in 2002, co-firing only accounted for 2.57% of the renewable electricity generation. However, co-firing rates nearly doubled every year until 2005. Producing 2533 GW h in 2005, co-firing accounted for 14.95% of the renewable electricity generation in the UK. Co-firing production remained level in 2006, but in the following years (2007–2009) co-firing production decreased steadily to 1625 GWh. However, in 2010 and 2011, co-firing production increased to a high of 2964 GWh. [
16] Another example is the city of Aarhus in Denmark, where there are two stations with a production of 150 and 350 MWe [
17]. The main fuel used is pulverized coal, and straw replaces 20%. At the same time, in the Netherlands, a wider effort has been made to develop the technology as several units operate in different cities with a capacity of 400 to 600 MWe [
13,
14]. The substitution rate varies; however, it moves at low levels between 4% and 8%. An important element is the type of biomass used; in addition to solid biomass, pellets, husk, and wood biomass are used. Finally, Poland is a country where biomass co-firing has been greatly developed [
13,
14]. There are three large plants, of which two have a capacity of 1800 MWe and a third has a capacity of 590 MWe. The replacement rate is 10%, and they mainly use sawdust, chips, and coffee shells.
Outside of Europe, there is a great growth in North America as well, with the most characteristic example being the city of Ontario in Canada, where there are seven power plants with a capacity of 150 to 500 MWe, where different types of biomass are used, mainly wood pellets, agricultural residues, and grain screening [
18].
As already mentioned, various types of biomass have been tested in co-firing applications. Forest and agricultural residues, wood biomass, solid and waste biomass, and wood pellets are the most common types of biomass used in co-firing applications. In addition, husk, grains, plant biomass, wood chips, and olive kernels are combustible biomass materials that are tested and can be more efficient if they first undergo upgrade processes such as torrefaction [
19]. It should be noted that there are other biomass materials such as hazelnut shells [
20], fruit pellets [
21], lignocellulosic plants, and algae biomass that are most effective when used in gasification processes [
22,
23].The main criteria for selecting a type of biomass is its price and its availability. For example, the Nordic countries use forest biomass as large areas of forest cover their spatial boundaries, while in many industrial areas, waste biomass is used as there are large amounts of industrial waste. Low availability and high costs are the main reasons that sunflower husk pellets are not widely used. However, they have been tested in the laboratory mainly to test their effectiveness and possible problems that their use as a fuel can create [
24,
25,
26].
It is well known that cement industries are using waste-based biomass (RDF, used tires, sludge, etc.) as alternative fuels in rotary kilns, mostly for clinker production. In Heidelberg cement, the waste-based biomass used, which accounted for around 42% of the alternative fuel mix in 2021, makes a special contribution as it is considered climate-neutral under European legislation [
27]. Additionally, LafargeHolcim [
28], through a circular approach, wants to reduce the carbon intensity of its cement by substituting fossil fuels with pre-treated non-recyclable and biomass waste fuels to operate its cement kilns. Currently they aim to increase thermal substitution of biomass from 20.9% to 37% by 2030.
Of course, co-firing conditions found in the cement or lime industry cannot be compared to the conditions realized in GM and in magnesia sector in general due to the type of fuels (usually in the cement industry are preferred low cost fuels such as RDF, sludge, and others) and level of temperatures (lower temperatures are anticipated in comparison with GMs in cases where a range from 1100 to 1900 °C or higher is expected).
The emissions from life cycle assessment (LCA) for the production of MgO vary depending on the production route and fuel. Depending on the characteristics of the production process, total emissions can vary up to +/−1.17 tons of CO
2 per ton of MgO. The company RHI-AG in Austria produces MgO based on MgCO
3 with a rotary kiln and natural gas with emission of 1.34 CO
2 per ton of MgO. In comparison, the production using a shaft kiln and hard coal as fuel leads to emissions up to 2.51 tons of CO
2 per ton of MgO [
29]. A comparison focusing only on the applied process fuel (e.g., pet coke, natural gas, and others) shows that fuel emissions can vary between 0.4 and 1.3 tons of CO
2 per ton of MgO [
30]. This means that the greatest prospects, as well as requirements in terms of reducing emissions, are in the types of fuels used to produce the required energy.
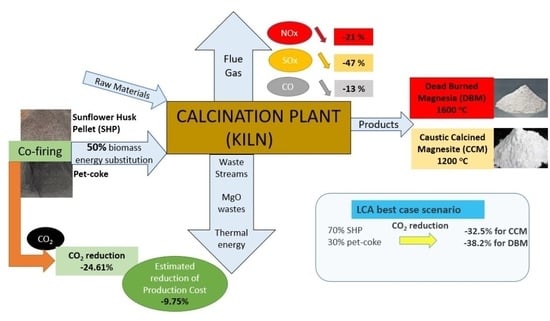
In this paper, an experimental campaign is presented with the main characteristic being the co-firing of a sunflower husk pellets/pet coke mixture in the new low-NO
x burner of the Yerakini Mine site calcination plant of Grecian Magnesite (GM) [
31]. An LCA is performed to assess the GHG reduction potential of various biomass feedstocks and mixtures with pet coke. Co-firing of fossil and biomass fuel is expected to lower greenhouse gas (GHG) emissions in the magnesia sector, hence playing a major role in sustainable MgO production. This research, both experimentally and theoretically, builds the foundation of future co-firing developments and improvements in the magnesia sector. The novelty of the paper lies in the fact that for the first time, the application of a fossil fuel/biomass co-firing process (with 50% energy substitution), in combination with the operation of an LNB burner, is being tested on a practical level in magnesia sector in order to reduce emissions and associated costs in a production process that has special requirements, such as large quantities of energy, extremely high temperatures (up to 1900 °C or higher), and specific strict properties for its final products (CCM, DBM).
2. Materials and Methods
2.1. Description of GMs Facilities and Use of Biomass
GM is a private company established in 1959 as a mining and industrial company that produces and trades CCM (caustic calcined magnesia), DBM (dead-burned magnesia), carbonate magnesium (MgCO3 –raw magnesite), and basic monolithic refractories. GM is listed as one of the top magnesia producers and exporters worldwide. Especially for CCM, the company is a leading producer in terms of volume and applications. The produced MgO (magnesia) is well known for its bright white color (whiteness) resulting from the low percentage in iron and its low levels of heavy metals and trace elements. In addition, the magnesia product is low in lime and has a microcrystalline structure. The ore is mined via open pit method. It is then transformed into the final product through the following four stages:
- i.
Pre-beneficiation, where different types of impurities are sorted out from the ore;
- ii.
Main-beneficiation, where the material enters the main beneficiation stage, in which it either passes through camera sorting or a combination of dense media and magnetic separation stages depending on the desired chemistry of the kiln-feed magnesite;
- iii.
Calcination and sintering in which the magnesite is fired in the kiln to produce CCM or DBM. During calcination, MgCO3 is decomposes to MgO. In sintering, the decomposed material is fired up to 2000 °C;
- iv.
Final processing, where the product is crushed and classified in different sizes.
Figure 1 gives an overview of the calcination plant in Yerakini, while in
Figure 2, GM’s production flowsheet is presented.
There are three (3) rotary kilns (RK) with a calcination capacity of 550 tpd and one shaft kiln (
Figure 1). Kiln-feed magnesite is fired in the kilns to produce either caustic calcined magnesia (CCM, at about 900 °C) or dead-burned magnesia (DBM, at about 1900 °C), using mostly pet coke as fuel. During calcination, magnesite (MgCO
3) is decomposed to magnesia by release of carbon dioxide according to the following reaction.
In the GM production process, petroleum coke (pet coke), heavy oil, and biomass are used as fuel. The firing process of magnesite into CCM/DBM produces large amounts of CO
2 and nitrogen oxides (NO
x) deriving from fuel combustion. There are two types of CO
2 emissions that are produced during this process: CO
2 produced from MgCO
3 decomposition, which is inevitable, and CO
2 emissions produced from fuel combustion. NO
x emissions produced during DBM production, where a 2000 °C temperature is needed, are also inevitable. In order to reduce the CO
2 emissions produced from fuel combustion, GM is substituted for a percentage of pet-coke energy with pulverized biomass (
Figure 3) in the form of sunflower husk pellets, olive cake, or sawdust, according to their seasonal availability and prices. In the BAMBOO project [
32], a novel and versatile low NO
x burner (LNB) was implemented by GM in order to reduce the respective emissions for temperatures up to 1600 °C but also to be able to reproduce high enough temperatures for the production of DBM. The required versatility is related to the fact that the new burner (~20 MWth) must have the ability to combust mixtures of pulverized biomass and pet coke and that it must have adjustable swirl to operate as an LNB for temperatures up to 1600 °C and in normal operation mode for DBM production (above 1600 °C). To optimize the combustion conditions of the raw material (raw magnesite), a small amount of wood chips (about 2–4% of pet coke) is also fired along with raw magnesite.
The potential of use wood chips as a feedstock in parallel with the magnesite gives the advantage of in situ and simultaneously De-NO
x and De-SO
x procedure. Feeding of wood chips along with raw magnesite (
Figure 4) can reduce NO
x emissions due to NO
2 reaction with the carbon (C) from wood chips and production of CO
2 and N
2 (2C+2NO
2 2CO
2+N
2 ). The overall benefits of the wood chip feedstock are expected to be: (a) NO
x (mainly NO
2) reduction, (b) SO
x reduction, and (c) preparation of the material.
2.2. Experimental Campaign in GM
After the installation of the new LNB in Rotary Kiln No.3 and the successful initial operation trials with 100% pet coke as fuel, GM conducted preliminary biomass/pet coke co-feeding trials with its initial preparation and feeding system (April–June 2021). Results were promising, but due to feeding system limitations, the trials faced problems caused by feeding instabilities. GM went on to design and construct a new system able to handle the new fuel mixture and conducted a successful experimental campaign in February of 2022.
Figure 5 describes the new fuel mix preparation and feed process designed and constructed by GM for the purposes of this trial. Biomass and pet coke are fed from the fuel temporary storage square adjacent to the calcination department facilities to two separate twin rotor hammer mills, and after size comminution, they are transported pneumatically to the solid fuel silo. From there they are conveyed by a screw feeder to a smaller buffer silo which assures further homogenization of the mixture and enables control of the mass feed rate of the mix to the new burner.
GM conducted initially a 4-day trial (100% pet coke) to validate the NOx reduction accomplished with the installation of the new burner that earlier shorter trials had indicated. Pet coke feed rate was 2000 kg/h with production of the base-case DBM product. Burner swirl was adjusted to the maximum levels for both inlet and outlet air.
After the 100% pet coke trial, without changing the kiln’s product and productivity, GM conducted a 5-day trial (co-firing with biomass) using a biomass/pet coke fuel mixture. The biomass used was sunflower husk pellet comprising 2/3 of the mixture by weight, or around 50% of the energy requirement. Burner swirls were kept at the maximum levels as before.
2.3. Description of Metering Equipment Used in Campaign
A dedicated control set-up, with the use of SCADA system, controls the operation of the feeding unit. Set point is a given mass flow rate (kg/h), which is achieved by adjusting screw speed. What is actually measured is the buffer silo weight with respect to time. A series of kiln parameters are controlled and monitored, the most important of which are the raw magnesite feed rate, rotation speed (as % of maximum), and temperature profile.
Flue gas composition is monitored by a SICK’s MCS100FT FTIR analyzer system (located in the stack of desulfurization unit) coupled with a flame ionization detector, a zirconium dioxide sensor, and backward light scattering systems able to monitor SO2, NO2, CO, HCl, HF, H2O and TOC, O2, and dust.
Spot measurements at various points for SO2, NO2, CO, and O2 are made with a portable, heavy-duty Varioplus Industrial by MRU, suitable for industrial applications by means of infrared technology (combination of NDIR technology with electrochemical sensors).
2.4. Description of Laboratory Equipment Used for Fuel Analysis
Several tests were conducted on pet coke and sunflower husk fuels and wood chips, including proximate analysis, ultimate analysis, determination of major and minor elements, bulk density and determination of ash melting temperature and calorific value.
Total moisture was measured using a furnace type Heraeus Thermo Scientific T-12 (temperature temporal deviation of ±5 °C). The measurement of moisture, ash, and volatiles was carried out in a Thermo Gravimetric Analysis (TGA ELTRA Thermostep, temperature control precision of 2% or ±2 °C).
The calorific values of the fuels were determined using a Parr 6400CL Calorimeter (relative standard deviation below or equal to 0.10%). The elemental analysis (CHN) was conducted using a Perkin Elmer Series II instrument (accuracy below 0.3%).
The concentration of major elements and selected heavy metals was determined by means of Flame and Graphite Furnace Atomic Absorption Spectrometry (AAS, Shimatzu AA-6300, relative standard deviation below 0.5%) after the complete digestion of samples with an acid mixture of HNO3/H2O/HF in a microwave oven (Berghof SW-2).
Ash fusion temperatures were measured in an oxidizing environment in a SYLAB IF2000G analyzer (precision better than ±20 °C on specific points). Fusion of ash is characterized by the physical state of the ash, which occurs during the heating process under well-defined conditions in the furnace. During ash fusion, the following temperatures were monitored:
Shrinkage temperature (ST): the temperature at which shrinking of the test piece occurs. This temperature is defined as when the area of the test piece falls below 95% of the original test piece area.
Deformation temperature (DT): the temperature at which the first signs of rounding of the edge of the test piece occurs due to melting.
Hemisphere temperature (HT): the temperature at which the test piece forms approximately a hemisphere, i.e., when the height becomes equal to half of the base diameter.
Flow temperature (FT): the temperature at which the ash is spread out over the supporting tile in a layer, the height of which is held of the height of the test piece at the hemisphere temperature.
During the laboratory analyses, the measurement processes and standards were strictly followed.
Table 1 records the processes as well as the standards that were followed in the laboratory facilities both during the analysis of the fuel (pet coke) and during the analysis of the different types of biomass. More information on the measuring instruments is presented in
Appendix A.
2.5. Description of LCA Methodology and Developed LCA Model
The environmental impacts are examined using the LCA software GaBi 10.6 ts by Sphera, following the ISO 14040 standards for LCA. This framework consists of four steps: definition of goal and scope, life cycle inventory (LCI), life cycle impact assessment (LCIA), and, finally, improvement and interpretation [
33]. As the LCA methodology is already extensively described in the literature [
34,
35,
36], only the relevant definitions to conduct the LCA are described.
2.5.1. Definition of Goal and Scope
The goal is to analyze the environmental impacts resulting from the use of biomass as alternative fuel compared to a business-as-usual (BAU) case. As elaborated previously [
37], the definition of a functional unit is crucial to guarantee comparability of alternatives. In this study, the functional unit is the production of 1 ton of MgO, either CCM or DBM. A cradle-to-gate system boundary for the MgO production plant is chosen according to the LCA framework. This means that the transport of the resources (pet coke, MgCO
3, biomass resources, etc.) and the production of MgO is considered within the analysis, but the utilization and transport of MgO is not part of the analysis.
The reference system is defined as the BAU system. The BAU case uses fossil pet coke for the kiln and calcination stage as fuel for thermal process energy provision and an electricity consumption mix for Greece as electricity supply. As the thermal energy demand is different for CCM and DBM, the LCA is conducted for CCM and DBM separately concerning the representative input data but is analogously related to applied methods.
2.5.2. Life Cycle Inventory and data collection
The life cycle inventory (LCI) phase focuses on data collection and quantifies the inputs and outputs of the production system. The mass and energy balance data for MgO production are collected based on [
33] (
Table 2 and
Table 3), and data gaps are filled using valid literature data. For the evaluation of the environmental impacts of the MgCO
3 mining process, representative literature values for open mining are applied [
38]. As the transportation of the MgCO
3 to the MgO production plant is not considered in the literature, this is included separately.
In contrast, the background processes, such as electricity generation or material production, were taken from acknowledged LCA databases, such as GaBi ts 10.6 Professional Database and ecoinvent v.3.8 database.
The electricity used for the production process of MgO is consumed from the public grid of Greece. In addition, a future renewable energy (RES) mix for Greece is composed using Greek data from GaBi LCA software. The production of 1 MJ electricity consists equally of hydro power, wind, and photovoltaic power in this RES mix.
The applied biomass resources can be mostly considered as agricultural residues and thus no ecological footprint is allocated. This is in line with the renewable energy directive (RED) of the European Parliament and Council [
39]. However, energy and emissions resulting from the collection, clustering, chipping, and pelletizing of biomass have to be considered. The respective data reported by the Joint Research Center (JRC) of the European Commission is applied [
40].
The calcination of MgCO3 releases an essential amount of CO2 naturally bound in the mineral complexes. The production of 1 ton of MgO from pure MgCO3 generates 1.09 tons of CO2 (assuming a stoichiometric reaction), which is considered within the LCA. In addition, the combustion of 1 ton of carbon (C) generates 3.66 tons of CO2 (assuming again a stoichiometric reaction). Within the LCA model, 1 ton of pet coke is considered with a carbon content of 88% and is taken into account for emissions from fuel combustion.
2.5.3. Life Cycle Impact Assessment
LCI compilation follows by setting up the MgO production process as an LCA model in GaBits 10.6 software and conducting the LCIA, applying CML 2001 methodology. In general, the LCA analyzes the environmental impacts of the MgO production in several categories. The most discussed and crucial one in this study is the global warming potential (GWP). The GWP indicator is calculated in kg CO2-equivalents for the impact category climate change.
2.5.4. Scenario Development for CCM and DBM MgO LCA Analysis
Scenarios support identifying the impacts of various parameters.
Table 4 gives an overview of the defined scenarios for the LCA analysis valid for CCM and DBM. The scenarios differ into following issues:
The electricity supply is changed for a best case scenario from the Greek electricity consumption mix to a renewable energy sources (RES) mix for Greece.
At the kiln process stage, biomass resources substitute pet coke as fuel based on their LHV for thermal process energy supply. Applied biomass resources are wood chips (WC) and pruning (PRU).
At the calcination process stage, biomass resources substitute pet coke as fuel. Applied biomass resources are sunflower husk pellets (SHP), wood saw dust (WSD), and olive kernels (OK).
Table 4.
Scenario description for CCM and DBM MgO LCA analysis.
Table 4.
Scenario description for CCM and DBM MgO LCA analysis.
| Abbreviation | Scenario | Description |
|---|
| BAU | business as usual | fossil fuel supplied process, thermal energy from pet coke and electricity from GR electricity mix |
| ELE | only electricity | 100% use of renewable electricity sources, thermal energy from pet coke |
| KILN | only kiln | 10% WC biomass share at kiln, GR electricity mix |
| CAL SHP | only calcination SHP | 70% SHP biomass share at calcination, GR electricity mix |
| CAL WSD | only calcination WSD | 70% WSD biomass share at calcination, GR electricity mix |
| CAL OK | only calcination OK | 70% OK biomass share at calcination, GR electricity mix |
| MB SHP | moderate biomass SHP | biomass share: at kiln WC 5%, at calcination SHP 30%; GR electricity mix |
| MB WSD | moderate biomass WSD | biomass share: at kiln WC 5%, at calcination WSD 30%; GR electricity mix |
| MB OK | moderate biomass OK | biomass share: at kiln WC 5%, at calcination OK 30%; GR electricity mix |
| BCB SHP | best case biomass SHP | biomass share: at kiln WC 10%, at calcination SHP 70%; RES electricity mix |
| BCB WSD | best case biomass WSD | biomass share: at kiln WC 10%, at calcination WSD 70%; RES electricity mix |
| BCB OK | best case biomass OK | biomass share: at kiln WC 10%, at calcination OK 70%; RES electricity mix |
4. Conclusions
The implementation of new, innovative processes that contribute to the reduction of emitted pollutants is a requirement for every industry. GM, with the implementation of the LNB system, drastically reduced (over 40%) NOx emissions. At the same time, by substituting part of the fuel (pet coke) with biomass (SHP), NOx emissions were further reduced by 21%, while SOx and CO were reduced by 20% and 13%, respectively. The ratio of substitution was 2 to 1 by weight, which corresponds to a contribution of biomass to the energy potential of the final fuel that reaches 50%. Mixing the fuel with biomass varied its characteristics, reducing its calorific value and increasing the percentage of moisture and ash, which, however, did not create any problems in the operation and efficiency of the burner. However, the calculation of an empirical ash fusibility index (Rs) showed that mixture of pet coke with sunflower husk pellets presents a medium trend for deposit formulation, a fact that needs to be considered carefully by the company in combination with the assessment of actual deposit formulation after a long time of kiln operation.
The conducted comparison via life cycle assessment for 1 ton of MgO revealed that for both CCM (2.24 ton of CO
2eq) and DBM (2.65 ton of CO
2eq), the most influencing GWP factors are the decomposition of MgCO
3 and the carbon released by the combustion of pet coke. It turned out that the substitution of the fuel by biomass and the use of renewable electricity can significantly reduce both the emissions of gaseous pollutants and the general environmental footprint of the MgO production. In a best case scenario, emissions can be decreased by 32.5% for CCM to 1.51 ton of CO
2 and by 38.2% for DBM to 1.64 ton of CO
2 per ton of MgO. The MgCO
3 decomposition process releases an essential and unavoidable amount of CO
2 naturally bound in the mineral complexes. In addition, the combustion of pet coke still releases a high share on total CO
2 emissions. However, the scenario-based evaluation of different biomass mixtures and resources provided evidence for encouraging biomass fuel use in the production process to become sustainable and resilient against future increased CO
2 prices. The implementation of CCS and CCU technologies can be an additional option to lower the ecological footprint of produced MgO to reduce the actual significant environmental impact of global magnesium production [
58].
The effective application of co-firing technologies, especially in such operating conditions, is a big step towards reducing the emissions of industry sector while at the same time proving that the use of fossil fuels can be reduced without altering the functionality and efficiency of processes that require large amounts of energy, extremely high temperatures, and high quality of final products. The further substitution of pet coke with biomass (perhaps the complete substitution) as well as the development of more environmentally friendly burners are a subject of further study but also an attractive target for GM and corresponding industries in the magnesia sector.