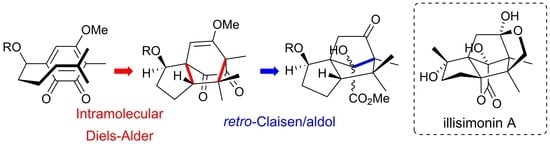
Synthesis of Illisimonin a Skeleton by Intramolecular Diels–Alder Reaction of Ortho-Benzoquinones and Biomimetic Skeletal Rearrangement of Allo-Cedranes
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukuyama, Y.; Huang, J.-M. Chemistry and Neurotrophic Activity of seco-Prezizaane- and Anislactone-type Sesquiterpenes from Illicium Species. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 32, pp. 395–427. [Google Scholar]
- Patra, J.K.; Das, G.; Bose, S.; Banerjee, S.; Vishnuprasad, C.N.; del Pilar Rodriguez-Torres, M.; Shin, H.-S. Star Anise (Illicium verum): Chemical Compounds, Antiviral Properties, and Clinical Relevance. Phytother. Res. 2020, 34, 1248–1267. [Google Scholar] [CrossRef]
- Witkin, J.M.; Shenvi, R.A.; Li, X.; Gleason, S.D.; Weiss, J.; Morrow, D.; Catow, J.T.; Wakulchik, M.; Ohtawa, M.; Lu, H.-H.; et al. Pharmacological Characterization of the Neurotrophic Sesquiterpene Jiadifenolide Reveals a Non-Convulsant Signature and Potential for Progression in Neurodegenerative Disease Studies. Biochem. Pharmacol. 2018, 155, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.F.; Koch, W.T.; Leeds, N.S.; Gorin, G. On the Toxin of Illicium Anisatum. I. The Isolation and Characterization of a Convulsant Principle: Anisatin. J. Am. Chem. Soc. 1952, 74, 3211–3215. [Google Scholar] [CrossRef]
- Yamada, K.; Takada, S.; Nakamura, S.; Hirata, Y. The Structures of Anisatin and Neoanisatin: Toxic Sesquiterpenes from Illicium Anisatum L. Tetrahedron 1968, 24, 199–229. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Shida, N.; Kodama, M. Tashironin, a plausible biosynthetic precursor of anisatin-type sesquiterpenes. Tetrahedron Lett. 1995, 36, 583–586. [Google Scholar] [CrossRef]
- Huang, J.-M.; Yokoyama, R.; Yang, C.-S.; Fukuyama, Y. Merrilactone A, a Novel Neurotrophic Sesquiterpene Dilactone from Illicium merrillianum. Tetrahedron Lett. 2000, 41, 6111–6114. [Google Scholar] [CrossRef]
- Huang, J.-M.; Yokoyama, R.; Yang, C.-S.; Fukuyama, Y. Structure and Neurotrophic Activity of seco-Prezizaane-Type Sesquiterpenes from Illicium merrillianum. J. Nat. Prod. 2001, 64, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Okada, C.; Huang, J.-M.; Harada, K.; Hioki, H.; Fukuyama, Y. Novel Pentacyclic seco-Prezizaane-Type Sesquiterpenoids with Neurotrophic Properties from Illicium jiadifengpi. Org. Lett. 2009, 11, 5190–5193. [Google Scholar] [CrossRef] [PubMed]
- Urabe, D.; Inoue, M. Total Syntheses of Sesquiterpenes from Illicium Species. Tetrahedron 2009, 65, 6271–6289. [Google Scholar] [CrossRef]
- Condakes, M.L.; Novaes, L.F.T.; Maimone, T.J. Contemporary Synthetic Strategies toward Seco-Prezizaane Sesquiterpenes from Illicium Species. J. Org. Chem. 2018, 83, 14843–14852. [Google Scholar] [CrossRef]
- Ogura, A.; Yamada, K.; Yokoshima, S.; Fukuyama, T. Total Synthesis of (−)-Anisatin. Org. Lett. 2012, 14, 1632–1635. [Google Scholar] [CrossRef]
- Xu, J.; Trzoss, L.; Chang, W.K.; Theodorakis, E.A. Enantioselective Total Synthesis of (−)-Jiadifenolide. Angew. Chem. Int. Ed. 2011, 50, 3672–3676. [Google Scholar] [CrossRef] [PubMed]
- Siler, D.A.; Mighion, J.D.; Sorensen, E.J. An Enantiospecific Synthesis of Jiadifenolide. Angew. Chem. Int. Ed. 2014, 53, 5332–5335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, I.; Xuan, M.; Dalby, S.M. Total Synthesis of Jiadifenolide. Angew. Chem. Int. Ed. 2014, 53, 7286–7289. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.H.; Martinez, M.D.; Shenvi, R.A. An eight-step gram-scale synthesis of (−)-jiadifenolide. Nat. Chem. 2015, 7, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, L.; Pan, Z.; Wang, Y.; Li, J.; Wang, K.; Wang, X.; Zhang, Y.; Hu, T.; Zhang, Y. Protecting-Group-Free Total Synthesis of (−)-Jiadifenolide: Development of a [4 + 1] Annulation toward Multisubstituted Tetrahydrofurans. Org. Lett. 2015, 17, 5480–5483. [Google Scholar] [CrossRef]
- Gomes, J.; Daeppen, C.; Liffert, R.; Roesslein, J.; Kaufmann, E.; Heikinheimo, A.; Neuburger, M.; Gademann, K. Formal Total Synthesis of (−)-Jiadifenolide and Synthetic Studies toward seco-Prezizaane-Type Sesquiterpenes. J. Org. Chem. 2016, 81, 11017–11034. [Google Scholar] [CrossRef] [Green Version]
- Mehta, G.; Maity, P. A Total Synthesis of 11-O-Methyldebenzoyltashironin. Tetrahedron Lett. 2011, 52, 1749–1752. [Google Scholar] [CrossRef]
- Ohtawa, M.; Krambis, M.J.; Cerne, R.; Schkeryantz, J.M.; Witkin, J.M.; Shenvi, R.A. Synthesis of (−)-11-O-Debenzoyltashironin: Neurotrophic Sesquiterpenes Cause Hyperexcitation. J. Am. Chem. Soc. 2017, 139, 9637–9644. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, P.; Yu, F.; Yang, Y.; Zhu, S.; Zhai, H. Total Synthesis of (±)-Merrilactone A. Angew. Chem. Int. Ed. 2012, 51, 5897–5899. [Google Scholar] [CrossRef]
- Liu, W.; Wang, B. Synthesis of (±)-Merrilactone A by a Desymmetrization Strategy. Chem.-Eur. J. 2018, 24, 16511–16515. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, L.; Xiao, X.; Yang, S.; Hua, Y.; Wang, Y.; Zhang, Y.; Zhang, Y. Site-Specific Photochemical Desaturation Enables Divergent Syntheses of Illicium Sesquiterpenes. J. Am. Chem. Soc. 2021, 143, 3256–3263. [Google Scholar] [CrossRef]
- Ma, S.-G.; Li, M.; Lin, M.-B.; Li, L.; Liu, Y.-B.; Qu, J.; Li, Y.; Wang, X.-J.; Wang, R.-B.; Xu, S.; et al. Illisimonin A, a Caged Sesquiterpenoid with a Tricyclo[5.2.1.01,6]decane Skeleton from the Fruits of Illicium simonsii. Org. Lett. 2017, 19, 6160–6163. [Google Scholar] [CrossRef]
- Burns, A.S.; Rychnovsky, S.D. Total Synthesis and Structure Revision of (−)-Illisimonin A, a Neuroprotective Sesquiterpenoid from the Fruits of Illicium simonsii. J. Am. Chem. Soc. 2019, 141, 13295–13300. [Google Scholar] [CrossRef]
- Gill, G.B. Benzil-Benzilic acid Rearrangements. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 3, pp. 821–838. [Google Scholar]
- Deb, S.; Chakraborti, R.; Ghatak, U.R. Synthesis of 1-p-Methoxyphenyl and 1-p-Methoxyphenyl-4-methylbicyclo[2.2.1]heptan-7-one. The Oxidation of 7-Hydroxy-1-p-methoxyphenyl-4-methylbicyclo[2.2.1]heptan-7-carboxylic Acid with Lead Tetraacetate. Synth. Comm. 1993, 23, 913–924. [Google Scholar] [CrossRef]
- Song, L.; Zhu, G.; Liu, Y.; Liu, B.; Qin, S. Total Synthesis of Atisane-Type Diterpenoids: Application of Diels−Alder Cycloadditions of Podocarpane-Type Unmasked ortho-Benzoquinones. J. Am. Chem. Soc. 2015, 137, 13706–13714. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Wang, J.; Tang, Y.; Botta, L. Total Synthesis of Sporolide B and 9-epi-Sporolide B. J. Am. Chem. Soc. 2010, 132, 11350–11363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Q.-S.; Yang, D. Enantioselective Synthesis of (+)-Mitomycin K by a Palladium-Catalyzed Oxidative Tandem Cyclization. Angew. Chem. Int. Ed. 2017, 56, 5886–5889. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, Y.; Nakahara, S.; Numata, R.; Kubo, A. Synthesis of 4,7-Indolequinones. The Oxidative Demethylation of 4,7-Dimethoxyindoles with Ceric Ammonium Nitrate. Chem. Pharm. Bull. 1985, 33, 2122–2128. [Google Scholar] [CrossRef] [Green Version]
- Magauer, T.; Martin, H.J.; Mulzer, J. Ring-Closing Metathesis and Photo-Fries Reaction for the Construction of the Ansamycin Antibiotic Kendomycin: Development of a Protecting Group Free Oxidative Endgame. Chem. Eur. J. 2010, 16, 507–519. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Nagahama, R.; Fariz, M.A.; Yukutake, Y.; Ikeuchi, K.; Tanino, K. Synthesis of Illisimonin a Skeleton by Intramolecular Diels–Alder Reaction of Ortho-Benzoquinones and Biomimetic Skeletal Rearrangement of Allo-Cedranes. Organics 2021, 2, 306-312. https://doi.org/10.3390/org2030016
Suzuki T, Nagahama R, Fariz MA, Yukutake Y, Ikeuchi K, Tanino K. Synthesis of Illisimonin a Skeleton by Intramolecular Diels–Alder Reaction of Ortho-Benzoquinones and Biomimetic Skeletal Rearrangement of Allo-Cedranes. Organics. 2021; 2(3):306-312. https://doi.org/10.3390/org2030016
Chicago/Turabian StyleSuzuki, Takahiro, Riko Nagahama, Muhammad Aiman Fariz, Yuki Yukutake, Kazutada Ikeuchi, and Keiji Tanino. 2021. "Synthesis of Illisimonin a Skeleton by Intramolecular Diels–Alder Reaction of Ortho-Benzoquinones and Biomimetic Skeletal Rearrangement of Allo-Cedranes" Organics 2, no. 3: 306-312. https://doi.org/10.3390/org2030016
APA StyleSuzuki, T., Nagahama, R., Fariz, M. A., Yukutake, Y., Ikeuchi, K., & Tanino, K. (2021). Synthesis of Illisimonin a Skeleton by Intramolecular Diels–Alder Reaction of Ortho-Benzoquinones and Biomimetic Skeletal Rearrangement of Allo-Cedranes. Organics, 2(3), 306-312. https://doi.org/10.3390/org2030016