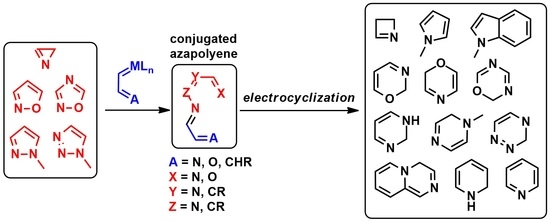
Electrocyclizations of Conjugated Azapolyenes Produced in Reactions of Azaheterocycles with Metal Carbenes
Abstract
:1. Introduction
2. 1,4-Electrocyclization of Azabutadienes
Electrocyclization of 2-Azabuta-1,3-dienes to 2,3-Dihydroazetes
3. 1,6- and 1,5-Electrocyclizations of Aza-, Oxaza-, Diaza-, and Oxadiazahexatrienes
3.1. 1,6-Electrocyclization of 1-Oxa-5-azahexa-1,3,5-trienes to 2H-1,3-Oxazines
3.2. 1,6-Electrocyclization of 1-Oxa-4-azahexa-1,3,5-trienes to 2H-1,4-Oxazines
3.3. 1,6-Electrocyclization of 1,5-Diazahexa-1,3,5-trienes to 1,2-Dihydropyrimidines
3.4. 1,6- and 1,5-Electrocyclizations of 1,4-Diazahexa-1,3,5-trienes
3.5. 1,6- and 1,5-Electrocyclizations of 2-Azahexa-1,3,5-trienes
3.6. 1,6-Electrocyclization of 3-Azahexa-1,3,5-trienes to Dihydropyridines
3.7. 1,6-Electrocyclization of 1-Oxa-3,5-diazahexa-1,3,5-trienes to 2H-1,3,5-Oxadiazines
3.8. 1,6-Electrocyclization of 1,4,5-Triazahexa-1,3,5-trienes to 3,4-Dihydro-1,2,4-triazines
4. Conclusions
Funding
Conflicts of Interest
References
- Vinogradov, M.G.; Turova, O.V.; Zlotin, S.G. Catalytic Asymmetric Aza-Diels−Alder Reaction: Pivotal Milestones and Recent Applications to Synthesis of Nitrogen-Containing Heterocycles. Adv. Synth. Catal. 2021, 363, 1466–1526. [Google Scholar] [CrossRef]
- Sarmah, M.M.; Prajapati, D. Aza-Diels–Alder Reaction: An Efficient Approach for Construction of Heterocycles. Curr. Org. Chem. 2014, 18, 1586–1620. [Google Scholar] [CrossRef]
- Masson, G.; Lalli, C.; Benohoud, M.; Dagousset, G. Catalytic Enantioselective [4 + 2]-Cycloaddition: A Strategy to Access Aza-Hexacycles. Chem. Soc. Rev. 2013, 42, 902–923. [Google Scholar] [CrossRef] [PubMed]
- Monbaliu, J.-C.M.; Masschelein, K.G.R.; Stevens, C.V. Electron-Deficient 1- and 2-Azabuta-1,3-dienes: A Comprehensive Survey of Their Synthesis and Reactivity. Chem. Soc. Rev. 2011, 40, 4708–4739. [Google Scholar] [CrossRef]
- Jayakumar, S.; Ishar, M.P.S.; Mahajan, M.P. Recent Advances in Synthetic Applications of Azadienes. Tetrahedron 2002, 58, 379–471. [Google Scholar] [CrossRef]
- Jiang, B.; Du, W.; Chen, Y.-C. Modified Cinchona Alkaloid-Catalysed Enantioselective [4 + 4] Annulations of Cyclobutenones and 1-Azadienes. Chem. Commun. 2020, 56, 7257–7260. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Laurent, G.; Retailleau, P.; Folléas, B.; Brayer, J.-L.; Masson, G. Highly Enantioselective Aza-Diels–Alder Reaction of 1-Azadienes with Enecarbamates Catalyzed by Chiral Phosphoric Acids. Angew. Chem. Int. Ed. 2013, 125, 11294–11297. [Google Scholar] [CrossRef]
- Wang, C.-S.; Li, T.-Z.; Cheng, Y.-C.; Zhou, J.; Mei, G.-J.; Shi, F. Catalytic Asymmetric [4 + 1] Cyclization of Benzofuran-Derived Azadienes with 3-Chlorooxindoles. J. Org. Chem. 2019, 84, 3214–3222. [Google Scholar] [CrossRef]
- Wan, Y.; Zheng, X.; Ma, C. Conjugated Dienyne-Imides as Robust Precursors of 1-Azatrienes for 6π Electrocyclizations to Furo[2,3-b]dihydropyridine Cores. Angew. Chem. Int. Ed. 2018, 57, 5482–5486. [Google Scholar] [CrossRef]
- Kwon, S.H.; Seo, H.-A.; Cheon, C.-H. Total Synthesis of Luotonin A and Rutaecarpine from an Aldimine via the Designed Cyclization. Org. Lett. 2016, 18, 5280–5283. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Wang, X.-G.; Shen, G.-D.; Yang, T.; Yang, Y.-H.; Li, J.; Yang, M.-Y.; Sun, H.-M.; Wei, J.-F. A 6π Azaelectrocyclization Strategy towards the 1,5,9-Triazacoronenes. Adv. Synth. Catal. 2020, 362, 1651–1656. [Google Scholar] [CrossRef]
- Ross, J.A.; Seiders, R.P.; Lemal, D.M. An extraordinarily facile sulfoxide automerization. J. Am. Chem. Soc. 1976, 98, 4325–4327. [Google Scholar] [CrossRef]
- Zhao, Z.; Wei, H.; Xiao, K.; Cheng, B.; Zhai, H.; Li, Y. Facile Synthesis of Pyridines from Propargyl Amines: Concise Total Synthesis of Suaveoline Alkaloids. Angew. Chem. Int. Ed. 2019, 58, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.F.; Larghi, E.L.; Kaufman, T.S. Synthesis of Polysubstituted 3-Methylisoquinolines through the 6π-Electron Cyclization/Elimination of 1-Azatrienes derived from 1,1-Dimethylhydrazine. Eur. J. Org. Chem. 2018, 2018, 5605–5614. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, H.Y.; Oh, K. Tandem Reaction Approaches to Isoquinolones from 2-Vinylbenzaldehydes and Anilines via Imine Formation–6π-Electrocyclization–Aerobic Oxidation Sequence. Org. Lett. 2020, 22, 474–478. [Google Scholar] [CrossRef]
- Tarzia, G.; Balsamini, C.; Spadoni, G.; Duranti, E. Alkyl 2-(diphenylmethyleneamino)acrylates in the synthesis of α-amino acids. Synthesis 1988, 1988, 514–517. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Arasappan, A.; Homback, W.J.; Huffman, J.C. Preparation and Wittig reactions of an α-keto amino acid derivative. Tetrahedron Lett. 1990, 31, 157–160. [Google Scholar] [CrossRef]
- Barluenga, J.; Tomas, M.; Ballesteros, A.; Gotor, V. An easy synthesis of electron-withdrawing substituted 2-aza-1,3-dienes and their 1,4-cycloaddition with enamines. J. Chem. Soc. Chem. Commun. 1987, 1195–1196. [Google Scholar] [CrossRef]
- Barluenga, J.; Ferrero, M.; Palacios, F. An efficient and mild conditions synthesis of 2-aza-1,3-dienes from phospha-λ5-azenes. Tetrahedron Lett. 1988, 29, 4863–4864. [Google Scholar] [CrossRef]
- Barluenga, J.; Ferrero, M.; Palacios, F. Reactivity and selectivity of N-vinylic λ5-phosphazenes towards electrophiles. Synthesis of 2-aza-1,3-dienes. J. Chem. Soc., Perkin Trans. 1990, 1, 2193–2197. [Google Scholar] [CrossRef]
- Barluenga, J.; Ferrero, M.; Palacios, F. A simple and efficient «One pot» synthesis of 2-aza-1,3-butadienes from N-vinylic λ5-phosphazenes. Tetrahedron Lett. 1990, 31, 3497–3500. [Google Scholar] [CrossRef]
- Polychronidou, V.; Krupp, A.; Strohmann, C.; Antonchick, A.P. Cascade aza-Wittig/6π-Electrocyclization in the Synthesis of 1,6-Dihydropyridines. Org. Lett. 2021, 23, 6024–6029. [Google Scholar] [CrossRef]
- Wei, H.; Li, Y.; Xiao, K.; Cheng, B.; Wang, H.; Hu, L.; Zhai, H. Synthesis of Polysubstituted Pyridines via a One-Pot Metal-Free Strategy. Org. Lett. 2015, 17, 5974–5977. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Miel, H.; Ring, A.; Slattery, C.N.; Maguire, A.R.; McKervey, M.A. Modern Organic Synthesis with α-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. [Google Scholar] [CrossRef]
- Khlebnikov, A.F.; Novikov, M.S.; Rostovskii, N.V. Advances in 2H-azirine chemistry: A seven-year update. Tetrahedron 2019, 75, 2555–2624. [Google Scholar] [CrossRef]
- Khlebnikov, A.F.; Novikov, M.S.; Amer, A.A. Reactions of 2H-azirines with carbenoids from diazo esters: Transformations of novel azirinium ylides. Tetrahedron Lett. 2004, 45, 6003–6006. [Google Scholar] [CrossRef]
- Khlebnikov, A.F.; Novikov, M.S.; Amer, A.A.; Kostikov, R.R.; Magull, J.; Vidovic, D. Azirinium ylides from alkoxycarbonylcarbenoids and 2H-azirines: Generation and transformations. Russ. J. Org. Chem. 2006, 42, 515–526. [Google Scholar] [CrossRef]
- Novikov, M.S.; Khlebnikov, A.F.; Rostovskii, N.V.; Tcyrulnikov, S.; Suhanova, A.A.; Zavyalov, K.V.; Yufit, D.S. Pseudopericyclic 1,5- versus pericyclic 1,4- and 1,6-electrocyclization in electron-poor 4-Aryl-2-azabuta-1,3-dienes: Indole synthesis from 2H-azirines and diazo compounds. J. Org. Chem. 2015, 80, 18–29. [Google Scholar] [CrossRef]
- Smetanin, I.A.; Novikov, M.S.; Agafonova, A.V.; Rostovskii, N.V.; Khlebnikov, A.F.; Kudryavtsev, I.V.; Terpilowski, M.A.; Serebriakova, M.K.; Trulioff, A.S.; Goncharov, N.V. A novel strategy for the synthesis of thermally stable and apoptosis-inducing 2,3-dihydroazetes. Org. Biomol. Chem. 2016, 14, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Novikov, M.S.; Smetanin, I.A.; Khlebnikov, A.F.; Rostovskii, N.V.; Yufit, D.S. Synthesis of electron-poor 4-halo-2-azabuta-1,3-dienes by Rh(II)-catalyzed diazo ester-azirine coupling. 2-Azabuta-1,3-diene-2,3-dihydroazete valence isomerism. Tetrahedron Lett. 2012, 53, 5777–5780. [Google Scholar] [CrossRef]
- Smetanin, I.A.; Novikov, M.S.; Rostovskii, N.V.; Khlebnikov, A.F.; Starova, G.L.; Yufit, D.S. 4-Halo-2-azabuta-1,3-dienes as intermediates in the rhodium carbenoid-initiated transformation of 2-halo-2H-azirines into 2,3-dihydroazetes and 2,5-dihydrooxazoles. Tetrahedron 2015, 71, 4616–4628. [Google Scholar] [CrossRef]
- Golubev, A.A.; Smetanin, I.A.; Agafonova, A.V.; Rostovskii, N.V.; Khlebnikov, A.F.; Starova, G.L.; Novikov, M.S. [2 + 1 + 1] Assembly of spiro β-lactams by Rh(II)-catalyzed reaction of diazocarbonyl compounds with azirines/isoxazoles. Org. Biomol. Chem. 2019, 17, 6821–6830. [Google Scholar] [CrossRef]
- Koronatov, A.N.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. One-pot synthesis of 3-(pyridin-2-yl)-2,3-dihydroazetes via Rh(II)-catalyzed reaction of diazoesters with trimethylsilyl-protected 2-(pyridin-2-yl)-2H-azirines. Chem. Heterocycl. Compd. 2019, 55, 1185–1189. [Google Scholar] [CrossRef]
- Maeda, S. Organic Photochromic and Thermochromic Compounds; Plenum Press: New York, NY, USA, 1999; Volume 1, Chapter 2; pp. 85–109. [Google Scholar]
- Minkin, V.I. Photo-, Thermo-, Solvato-, and Electrochromic Spiroheterocyclic Compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef]
- Barkauskas, M.; Martynaitis, V.; Šačkus, A.; Rotomskis, R.; Sirutkaitis, V.; Vengris, M. Ultrafast Dynamics of Photochromic Compound Based on Oxazine Ring Opening. Lith. J. Phys. 2008, 48, 231–242. [Google Scholar] [CrossRef]
- Manning, J.R.; Davies, H.M.L. Efficient route to 2H-1,3-oxazines through ring expansion of isoxazoles by rhodium carbenoids. Tetrahedron 2008, 64, 6901–6908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khlebnikov, A.F.; Novikov, M.S.; Gorbunova, Y.G.; Galenko, E.E.; Mikhailov, K.I.; Pakalnis, V.V.; Avdontceva, M.S. Isoxazolium N-ylides and 1-oxa-5-azahexa-1,3,5-trienes on the way from isoxazoles to 2H-1,3-oxazines. Beilstein J. Org. Chem. 2014, 10, 1896–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavyalov, K.V.; Novikov, M.S.; Khlebnikov, A.F.; Yufit, D.S. Rh2(OAc)4-Catalyzed Reaction of α-Diazocarbonyl Compounds with 2-Carbonyl-Substituted 2H-Azirines. Tetrahedron 2013, 69, 4546–4551. [Google Scholar] [CrossRef]
- Zavyalov, K.V.; Novikov, M.S.; Khlebnikov, A.F.; Pakalnis, V.V. Selective Syntheses of 2H-1,3-Oxazines and 1H-Pyrrol-3(2H)-ones via Temperature-Dependent Rh(II)-Carbenoid-Mediated 2H-Azirine Ring Expansion. Tetrahedron 2014, 70, 3377–3384. [Google Scholar] [CrossRef]
- Khlebnikov, V.A.; Novikov, M.S.; Khlebnikov, A.F.; Rostovskii, N.V. Rh(II)-Catalyzed reactions of 2H-azirines with ethyl 2-acyl-2-diazoacetates. Synthesis of novel photochromic oxazines. Tetrahedron Lett. 2009, 50, 6509–6511. [Google Scholar] [CrossRef]
- Rostovskii, N.V.; Novikov, M.S.; Khlebnikov, A.F.; Khlebnikov, V.A.; Korneev, S.M. Rh(II)-carbenoid mediated 2H-azirine ring-expansion as a convenient route to non-fused photo- and thermochromic 2H-1,4-oxazines. Tetrahedron 2013, 69, 4292–4301. [Google Scholar] [CrossRef]
- Rostovskii, N.V.; Novikov, M.S.; Khlebnikov, A.F.; Starova, G.L.; Avdontseva, M.S. Azirinium ylides from α-diazoketones and 2H-azirines on the route to 2H-1,4-oxazines: Three-membered ring opening vs 1,5-cyclization. Beilstein J. Org. Chem. 2015, 11, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Novikov, M.S.; Rostovskii, N.V.; Koronatov, A.N.; Zavyalov, K.V.; Zubakin, G.V.; Khlebnikov, A.F.; Starova, G.L. Synthesis of 1,2-Dihydropyrimidine-2-carboxylates via Regioselective Addition of Rhodium(II) Carbenoids to 2H-Azirine-2-carbaldimines. J. Org. Chem. 2017, 82, 13396–13404. [Google Scholar] [CrossRef]
- Koronatov, A.N.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. Rh(II)-Catalyzed Ring Expansion of Pyrazoles with Diazocarbonyl Compounds as a Method for the Preparation of 1,2-Dihydropyrimidines. J. Org. Chem. 2018, 83, 9210–9219. [Google Scholar] [CrossRef]
- Rostovskii, N.V.; Ruvinskaya, J.O.; Novikov, M.S.; Khlebnikov, A.F.; Smetanin, I.A.; Agafonova, A.V. Switchable Synthesis of Pyrroles and Pyrazines via Rh(II)-Catalyzed Reaction of 1,2,3-Triazoles with Isoxazoles: Experimental and DFT Evidence for the 1,4-Diazahexatriene Intermediate. J. Org. Chem. 2017, 82, 256–268. [Google Scholar] [CrossRef]
- Zhao, Y.-Z.; Yang, H.-B.; Tang, X.-Y.; Shi, M. RhII-Catalyzed [3+2] Cycloaddition of 2H-Azirines with N-Sulfonyl-1,2,3-Triazoles. Chem. Eur. J. 2015, 21, 3562–3566. [Google Scholar] [CrossRef] [PubMed]
- Ryu, T.; Baek, Y.; Lee, P.H. Synthesis of Pyrazines from Rhodium-Catalyzed Reaction of 2H-Azirines with N-Sulfonyl 1,2,3-Triazoles. J. Org. Chem. 2015, 80, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lei, X.; Tang, Y. Rh(II)-Catalyzed Cycloadditions of 1-Tosyl-1,2,3-triazoles with 2H-Azirines: Switchable Reactivity of Rh-Azavinylcarbene as [2C]- or Aza-[3C]-Synthon. Chem. Commun. 2015, 51, 4507–4510. [Google Scholar] [CrossRef]
- Loy, N.S.Y.; Kim, S.; Park, C.-M. Synthesis of Unsymmetrical Pyrazines Based on α Diazo Oxime Ethers. Org. Lett. 2015, 17, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, Z.; Bai, S.; Lu, P.; Wang, Y. Rh-Catalyzed Conversion of 3-Diazoindolin-2-imines to 5H-Pyrazino[2,3-b]indoles with Photoluminescent Properties. Org. Lett. 2017, 19, 6514–6517. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Maeng, C.; Kim, H.; Lee, P.H. Regioselective Synthesis of Indolopyrazines through a Sequential Rhodium-Catalyzed Formal [3+3] Cycloaddition and Aromatization Reaction of Diazoindolinimines with Azirines. J. Org. Chem. 2018, 83, 2349–2360. [Google Scholar] [CrossRef]
- Ruvinskaya, J.O.; Rostovskii, N.V.; Filippov, I.P.; Khlebnikov, A.F.; Novikov, M.S. A Novel Approach to 5H-Pyrazino[2,3-b]indoles via Annulation of 3-Diazoindolin-2-imines with 2H-Azirines or 5-Alkoxyisoxazoles under Rh(II) Catalysis. Org. Biomol. Chem. 2018, 16, 38–42. [Google Scholar] [CrossRef]
- Filippov, I.P.; Novikov, M.S.; Khlebnikov, A.F.; Rostovskii, N.V. Pseudopericyclic Dearomative 1,6-Cyclization of 1-(2-Pyridyl)-2-azabuta-1,3-dienes: Synthesis and Ring–Chain Valence Equilibria of 4H-Pyrido[1,2-a]pyrazines. Eur. J. Org. Chem. 2020, 2020, 3688–3698. [Google Scholar] [CrossRef]
- Zavyalov, K.V.; Novikov, M.S.; Khlebnikov, A.F.; Rostovskii, N.V.; Starova, G.L. Rh2(OAc)4-catalyzed Reaction of 2-(2-Carbonylvinyl)-3-phenyl-2H-azirines with Diazo Esters. Russ. J. Org. Chem. 2017, 53, 1214–1221. [Google Scholar] [CrossRef]
- Zavyalov, K.V.; Novikov, M.S.; Khlebnikov, A.F.; Rostovskii, N.V. (3Z)-2-Azahexa-1,3,5-trienes: Generation and Regioselectivity of 1,5- and 1,6-Cyclizations. Russ. J. Org. Chem. 2016, 52, 1851–1853. [Google Scholar] [CrossRef]
- Khaidarov, A.R.; Rostovskii, N.V.; Zolotarev, A.A.; Khlebnikov, A.F.; Novikov, M.S. Synthesis of 1-(2-Aminovinyl)indoles and 1,3′-Biindoles by Reaction of 2,2-Diaryl-Substituted 2H-Azirines with α-Imino Rh(II) Carbenoids. J. Org. Chem. 2019, 84, 3743–3753. [Google Scholar] [CrossRef]
- Khaidarov, A.R.; Rostovskii, N.V.; Starova, G.L.; Khlebnikov, A.F.; Novikov, M.S. Synthesis of spirocyclic 3H-pyrrol-4-amines from 2H-azirines and 1-sulfonyl-1,2,3-triazoles. Chem. Heterocycl. Compd. 2018, 54, 946–950. [Google Scholar] [CrossRef]
- Loy, N.S.Y.; Singh, A.; Xu, X.; Park, C.-M. Synthesis of Pyridines by Carbenoid-Mediated Ring Opening of 2H-Azirines. Angew. Chem. Int. Ed. 2013, 52, 2212–2216. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-T.; Wang, Q.; Zhao, J.; Liu, X.-Y.; Xu, P.-F.; Liang, Y.-M. The copper-catalyzed synthesis of β-trifluoromethylated acrylonitriles and trifluoromethyl-substituted 2H-azirines. Chem. Commun. 2015, 51, 13209–13212. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.R.; Davies, H.M.L. One-Pot Synthesis of Highly Functionalized Pyridines via a Rhodium Carbenoid Induced Ring Expansion of Isoxazoles. J. Am. Chem. Soc. 2008, 130, 8602–8603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strelnikova, J.O.; Rostovskii, N.V.; Khoroshilova, O.V.; Khlebnikov, A.F.; Novikov, M.S. An Efficient Synthesis of Functionalized 2H-1,3,5-Oxadiazines via Metal-Carbenoid-Induced 1,2,4-Oxadiazole Ring Cleavage. Synthesis 2021, 53, 348–358. [Google Scholar]
- Koronatov, A.N.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. Synthesis of 3-Alkoxy-4-Pyrrolin-2-ones via Rhodium(II)-Catalyzed Denitrogenative Transannulation of 1H-1,2,3-Triazoles with Diazo Esters. Org. Lett. 2020, 22, 7958–7963. [Google Scholar] [CrossRef] [PubMed]
- Koronatov, A.N.; Afanaseva, K.K.; Sakharov, P.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. Rh(II)-Catalyzed denitrogenative 1-sulfonyl-1,2,3-triazole–1-alkyl-1,2,3-triazole cross-coupling as a route to 3-sulfonamido-1H-pyrroles and 1,2,3-triazol-3-ium ylides. Org. Chem. Front. 2021, 8, 1474–1481. [Google Scholar] [CrossRef]








































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostovskii, N.V.; Novikov, M.S.; Khlebnikov, A.F. Electrocyclizations of Conjugated Azapolyenes Produced in Reactions of Azaheterocycles with Metal Carbenes. Organics 2021, 2, 313-336. https://doi.org/10.3390/org2030017
Rostovskii NV, Novikov MS, Khlebnikov AF. Electrocyclizations of Conjugated Azapolyenes Produced in Reactions of Azaheterocycles with Metal Carbenes. Organics. 2021; 2(3):313-336. https://doi.org/10.3390/org2030017
Chicago/Turabian StyleRostovskii, Nikolai V., Mikhail S. Novikov, and Alexander F. Khlebnikov. 2021. "Electrocyclizations of Conjugated Azapolyenes Produced in Reactions of Azaheterocycles with Metal Carbenes" Organics 2, no. 3: 313-336. https://doi.org/10.3390/org2030017
APA StyleRostovskii, N. V., Novikov, M. S., & Khlebnikov, A. F. (2021). Electrocyclizations of Conjugated Azapolyenes Produced in Reactions of Azaheterocycles with Metal Carbenes. Organics, 2(3), 313-336. https://doi.org/10.3390/org2030017