Preoperative Nutritional Optimization and Physical Exercise for Patients Scheduled for Elective Implantation for a Left-Ventricular Assist Device—The PROPER-LVAD Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Registration and Ethics
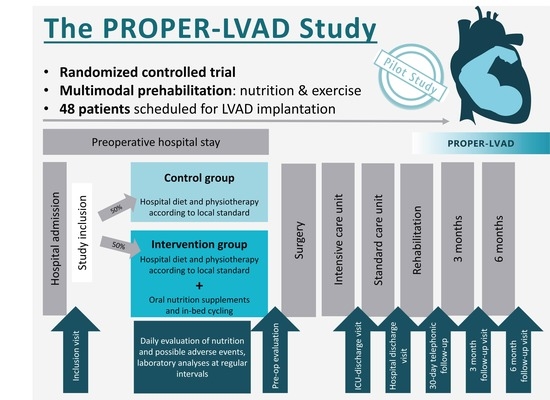
2.2. Study Design and Sites
- Phase 1 is a non-randomized run-in phase, where only ONS are administered to 8 patients. This phase evaluates the safety of the ONS therapy alone in this group of hemodynamically compromised patients.
- Phase 2 is a randomized controlled clinical trial, where the full study protocol is administered to 40 patients. In Phase 2, patients are either randomized to the full study protocol, consisting of ONS + in-bed cycling, or to the control group (regular hospital diet and physiotherapy as per standard protocol only).
2.3. Patients
2.4. Study Intervention
2.4.1. Nutrition Intervention and Monitoring
- In the unlikely event that the patient is discharged from the hospital prior to LVAD implantation, the study ONS will be stopped at the day of discharge and further nutrition therapy will be left to the discretion of the following facility.
- If a single dose of ONS was failed to be administered to the patient, it can be made up for the next day, since it is the average protein and energy intake that will allow the patient to improve physical function and adverse effects, due to the fact that increasing the amount of ONS by a single unit on a day is not likely.
- If more than a single dose of ONS was failed to be administered to the patient, it shall not be made up for on the next day to avoid adverse effects for the patient.
- In case of gastrointestinal or metabolic intolerance, patients with fluid restriction and patients experiencing discomfort during the nutrition intervention, the dosage of ONS will be adjusted according to the clinical symptoms and blood work. This will allow the patients to consume one drink less per day than initially planned (e.g., 1 ONS/d in patients without nutritional risk or 2 ONS/d in patients with nutritional risk).
2.4.2. Exercise Intervention and Monitoring
- Severe dyspnea, dizziness, or syncope;
- New malign arrhythmias, new signs of myocardial ischemia;
- Non-physiological reactions of vital parameters, including the following:
- ○
- Heart rate > 120 bpm, or change of >20%;
- ○
- Blood pressure (BP) > 180 mmHg systolic BP or decrease by 20% in systolic or diastolic BP;
- ○
- Transcutaneous oxygen saturation < 85% or decrease by >10%.
- In the unlikely event that the patient is discharged from the hospital prior to LVAD implantation, the exercise will be stopped at day of discharge.
- If an exercise session was missed by the patient, it should not be made up for the next day.
- If an exercise intervention needs to be terminated early due to interruptions, it will be attempted to be completed during the same day, adding warm-up and cool-down phases to the remaining minutes of exercise.
2.5. Control Group
2.6. Study Duration
2.7. Outcomes
- Muscle mass (quadriceps muscle layer thickness);
- Muscle strength (handgrip strength, quadriceps strength);
- Function (6-min walk distance, Short Physical Performance Battery, Functional Status Score for the ICU, Manual Muscle Testing, Clinical Frailty Scale, Barthel Index, Lawton Instrumental and Katz Activities of Daily Living, and Mini Mental State Exam);
- Quality of life (Short Form 36).
3. Discussion and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Members, W.G.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.-P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar]
- Berg, T.; Tewarie, L.; Moza, A.; Zayat, R.; Autschbach, R.; Stoppe, C.; Goetzenich, A.; Benstoem, C. Anforderungen an die ambulante Versorgung nach Implantation eines ventrikularen Herzunterstutzungssystems. Herz 2019, 44, 257–264. [Google Scholar] [CrossRef]
- Laribi, S.; Aouba, A.; Nikolaou, M.; Lassus, J.; Cohen-Solal, A.; Plaisance, P.; Pavillon, G.; Jois, P.; Fonarow, G.C.; Jougla, E.; et al. Trends in death attributed to heart failure over the past two decades in Europe. Eur. J. Heart Fail. 2012, 14, 234–239. [Google Scholar] [CrossRef]
- Stoerk, S.; Handrock, R.; Jacob, J.; Walker, J.; Calado, F.; Lahoz, R.; Hupfer, S.; Klebs, S. Epidemiology of heart failure in Germany: A retrospective database study. Clin. Res. Cardiol. Off. J. Ger. Card. Soc 2017, 106, 913–922. [Google Scholar] [CrossRef]
- Beckmann, A.; Meyer, R.; Lewandowski, J.; Markewitz, A.; Gummert, J. German Heart Surgery Report 2020: The Annual Updated Registry of the German Society for Thoracic and Cardiovascular Surgery. Thorac. Cardiovasc. Surg. 2021, 69, 294–307. [Google Scholar] [CrossRef]
- Cotts, W.G.; McGee, E.C.; Myers, S.L.; Naftel, D.C.; Young, J.B.; Kirklin, J.K.; Grady, K.L. Predictors of hospital length of stay after implantation of a left ventricular assist device: An analysis of the INTERMACS registry. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2014, 33, 682–688. [Google Scholar] [CrossRef]
- Bottle, A.; Faitna, P.; Aylin, P.P.; Cowie, M.R. Five-year outcomes following left ventricular assist device implantation in England. Open Heart 2021, 8, e001658. [Google Scholar] [CrossRef]
- Czermak, T.; Seitelberger, V.; Hagl, C.; Samson-Himmelstjerna, P.N.; Gross, S.; Sadoni, S.; Heyn, O.; Kellnar, A.; Hartrampf, B.; Lemmermohle, E.; et al. Survival after left ventricular assist device implantation correlates with a novel device-based measure of heart rate variability: The heart rate score. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 309–315. [Google Scholar] [CrossRef]
- Warren, O.J.; Smith, A.J.; Alexiou, C.; Rogers, P.L.B.; Jawad, N.; Vincent, C.; Darzi, A.W.; Athanasiou, T. The inflammatory response to cardiopulmonary bypass: Part 1-mechanisms of pathogenesis. J. Cardiothorac. Vasc. Anesth. 2009, 23, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Wragge, T.; Pasque, C. Determinants of mortality and multiorgan dysfunction in cardiac surgery patients requiring prolonged mechanical ventilation. Chest 1995, 107, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; McDonald, B.; Benstoem, C.; Elke, G.; Meybohm, P.; Whitlock, R.; Fremes, S.; Fowler, R.; Lamarche, Y.; Jiang, X.; et al. Evaluation of Persistent Organ Dysfunction Plus Death As a Novel Composite Outcome in Cardiac Surgical Patients. J. Cardiothorac. Vasc. Anesth. 2016, 30, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Nesterova, E.; Lomivorotov, V.; Efremov, S.; Goetzenich, A.; Benstoem, C.; Zamyatin, M.; Chourdakis, M.; Heyland, D.; Stoppe, C. Current Evidence about Nutrition Support in Cardiac Surgery Patients-What Do We Know? Nutrients 2018, 10, 597. [Google Scholar] [CrossRef]
- Hill, A.; Arora, R.C.; Engelman, D.T.; Stoppe, C. Preoperative Treatment of Malnutrition and Sarcopenia in Cardiac Surgery: New Frontiers. Crit. Care Clin. 2020, 36, 593–616. [Google Scholar] [CrossRef]
- Hoogeboom, T.J.; Dronkers, J.J.; Hulzebos, E.H.J.; van Meeteren, N.L.U. Merits of exercise therapy before and after major surgery. Curr. Opin. Anaesthesiol. 2014, 27, 161–166. [Google Scholar] [CrossRef]
- Grover, A.; Gorman, K.; Dall, T.M.; Jonas, R.; Lytle, B.; Shemin, R.; Wood, D.; Kron, I. Shortage of cardiothoracic surgeons is likely by 2020. Circulation 2009, 120, 488–494. [Google Scholar] [CrossRef]
- Humphrey, R.; Malone, D. Effectiveness of preoperative physical therapy for elective cardiac surgery. Phys. Ther. 2015, 95, 160–166. [Google Scholar] [CrossRef]
- Han, J.J.; Acker, M.A.; Atluri, P. Left Ventricular Assist Devices. Circulation 2018, 138, 2841–2851. [Google Scholar] [CrossRef]
- Parry, S.M.; Huang, M.; Needham, D.M. Evaluating physical functioning in critical care: Considerations for clinical practice and research. Crit. Care 2017, 21, 249. [Google Scholar] [CrossRef]
- Silberman, S.; Bitran, D.; Fink, D.; Tauber, R.; Merin, O. Very prolonged stay in the intensive care unit after cardiac operations: Early results and late survival. Ann. Thorac. Surg. 2013, 96, 15–21; discussion 21–22. [Google Scholar] [CrossRef] [PubMed]
- Paccagnella, A.; Calò, M.A.; Caenaro, G.; Salandin, V.; Jus, P.; Simini, G.; Heymsfield, S.B. Cardiac cachexia: Preoperative and postoperative nutrition management. J. Parenter. Enter. Nutr. 1994, 18, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Tepaske, R.; Velthuis, H.; Oudemans-van Straaten, H.M.; Heisterkamp, S.H.; van Deventer, S.J.; Ince, C.; Eysman, L.; Kesecioglu, J. Effect of preoperative oral immune-enhancing nutritional supplement on patients at high risk of infection after cardiac surgery: A randomised placebo-controlled trial. Lancet 2001, 358, 696–701. [Google Scholar] [CrossRef]
- Aquilani, R.; Opasich, C.; Gualco, A.; Verri, M.; Testa, A.; Pasini, E.; Viglio, S.; Iadarola, P.; Pastoris, O.; Dossena, M.; et al. Adequate energy-protein intake is not enough to improve nutritional and metabolic status in muscle-depleted patients with chronic heart failure. Eur. J. Heart Fail. 2008, 10, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Rozentryt, P.; von Haehling, S.; Lainscak, M.; Nowak, J.U.; Kalantar-Zadeh, K.; Polonski, L.; Anker, S.D. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: A randomized, double-blind pilot study. J. Cachexia Sarcopenia Muscle 2010, 1, 35–42. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–562. [Google Scholar] [CrossRef]
- LeBlanc, A.D.; Schneider, V.S.; Evans, H.J.; Pientok, C.; Rowe, R.; Spector, E. Regional changes in muscle mass following 17 weeks of bed rest. J. Appl. Physiol. 1992, 73, 2172–2178. [Google Scholar] [CrossRef]
- Griffiths, R.D.; Palmer, T.E.; Helliwell, T.; MacLennan, P.; MacMillan, R.R. Effect of passive stretching on the wasting of muscle in the critically ill. Nutrition 1995, 11, 428–432. [Google Scholar]
- Preiser, J.; Prato, C.; Harvengt, A.; Peters, L.; Bastin, M. Passive cycling limits myofibrillar protein catabolism in unconscious patients: A pilot study. J. Nov. Physiother. 2014, 4, 225. [Google Scholar]
- Flynn, K.E.; Pina, I.L.; Whellan, D.J.; Lin, L.; Blumenthal, J.A.; Ellis, S.J.; Fine, L.J.; Howlett, J.G.; Keteyian, S.J.; Kitzman, D.W.; et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009, 301, 1451–1459. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Whellan, D.J.; Lee, K.L.; Keteyian, S.J.; Cooper, L.S.; Ellis, S.J.; Leifer, E.S.; Kraus, W.E.; Kitzman, D.W.; Blumenthal, J.A.; et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009, 301, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Kozarez, I.; Adams, V.; Mangner, N.; Hoellriegel, R.; Erbs, S.; Linke, A.; Moebius-Winkler, S.; Thiery, J.; Kratzsch, J.; et al. Age-related effects of exercise training on diastolic function in heart failure with reduced ejection fraction: The Leipzig Exercise Intervention in Chronic Heart Failure and Aging (LEICA) Diastolic Dysfunction Study. Eur. Heart J. 2012, 33, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Lenk, K.; Erbs, S.; Hoellriegel, R.; Beck, E.; Linke, A.; Gielen, S.; Winkler, S.M.; Sandri, M.; Hambrecht, R.; Schuler, G.; et al. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur. J. Prev. Cardiol. 2012, 19, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hoellriegel, R.; Beck, E.B.; Linke, A.; Adams, V.; Moebius-Winkler, S.; Mangner, N.; Sandri, M.; Gielen, S.; Gutberlet, M.; Hambrecht, R.; et al. Anabolic effects of exercise training in patients with advanced chronic heart failure (NYHA IIIb): Impact on ubiquitin-protein ligases expression and skeletal muscle size. Int. J. Cardiol. 2013, 167, 975–980. [Google Scholar] [CrossRef]
- Arthur, H.M.; Daniels, C.; McKelvie, R.; Hirsh, J.; Rush, B. Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann. Intern. Med. 2000, 133, 253–262. [Google Scholar] [CrossRef]
- Herdy, A.H.; Marcchi, P.L.B.; Vila, A.; Tavares, C.; Collaco, J.; Niebauer, J.; Ribeiro, J.P. Pre- and postoperative cardiopulmonary rehabilitation in hospitalized patients undergoing coronary artery bypass surgery: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2008, 87, 714–719. [Google Scholar] [CrossRef]
- Rosenfeldt, F.; Braun, L.; Spitzer, O.; Bradley, S.; Shepherd, J.; Bailey, M.; van der Merwe, J.; Leong, J.-Y.; Esmore, D. Physical conditioning and mental stress reduction--a randomised trial in patients undergoing cardiac surgery. BMC Complementary Altern. Med. 2011, 11, 20. [Google Scholar] [CrossRef]
- Tung, H.-H.; Shen, S.-F.; Shih, C.-C.; Chiu, K.-M.; Lee, J.-Y.; Liu, C.-Y. Effects of a preoperative individualized exercise program on selected recovery variables for cardiac surgery patients: A pilot study. J. Saudi Heart Assoc. 2012, 24, 153–161. [Google Scholar] [CrossRef]
- Burtin, C.; Clerckx, B.; Robbeets, C.; Ferdinande, P.; Langer, D.; Troosters, T.; Hermans, G.; Decramer, M.; Gosselink, R. Early exercise in critically ill patients enhances short-term functional recovery. Crit. Care Med. 2009, 37, 2499–2505. [Google Scholar] [CrossRef]
- Pires-Neto, R.C.; Kawaguchi, Y.M.F.; Hirota, A.S.; Fu, C.; Tanaka, C.; Caruso, P.; Park, M.; Carvalho, C.R.R. Very early passive cycling exercise in mechanically ventilated critically ill patients: Physiological and safety aspects-a case series. PLoS ONE 2013, 8, e74182. [Google Scholar] [CrossRef]
- Rahimi, R.A.; Skrzat, J.; Reddy, D.R.S.; Zanni, J.M.; Fan, E.; Stephens, R.S.; Needham, D.M. Physical rehabilitation of patients in the intensive care unit requiring extracorporeal membrane oxygenation: A small case series. Phys. Ther. 2013, 93, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kho, M.E.; Martin, R.A.; Toonstra, A.L.; Zanni, J.M.; Mantheiy, E.C.; Nelliot, A.; Needham, D.M. Feasibility and safety of in-bed cycling for physical rehabilitation in the intensive care unit. J. Crit. Care 2015, 30, 1419.e1–1419.e5. [Google Scholar] [CrossRef] [PubMed]
- Fossat, G.; Baudin, F.; Courtes, L.; Bobet, S.; Dupont, A.; Bretagnol, A.; Benzekri-Lefevre, D.; Kamel, T.; Muller, G.; Bercault, N.; et al. Effect of In-Bed Leg Cycling and Electrical Stimulation of the Quadriceps on Global Muscle Strength in Critically Ill Adults: A Randomized Clinical Trial. JAMA 2018, 320, 368–378. [Google Scholar] [CrossRef] [Green Version]
- Nickels, M.R.; Aitken, L.M.; Barnett, A.G.; Walsham, J.; King, S.; Gale, N.E.; Bowen, A.C.; Peel, B.M.; Donaldson, S.L.; Mealing, S.T.J.; et al. Effect of in-bed cycling on acute muscle wasting in critically ill adults: A randomised clinical trial. J. Crit. Care 2020, 59, 86–93. [Google Scholar] [CrossRef]
- Waldauf, P.; Hruskova, N.; Blahutova, B.; Gojda, J.; Urban, T.; Krajcova, A.; Fric, M.; Jiroutkova, K.; Rasova, K.; Duska, F. Functional electrical stimulation-assisted cycle ergometry-based progressive mobility programme for mechanically ventilated patients: Randomised controlled trial with 6 months follow-up. Thorax 2021, 76, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Berney, S.; Hopkins, R.O.; Rose, J.W.; Koopman, R.; Puthucheary, Z.; Pastva, A.; Gordon, I.; Colantuoni, E.; Parry, S.M.; Needham, D.M.; et al. Functional electrical stimulation in-bed cycle ergometry in mechanically ventilated patients: A multicentre randomised controlled trial. Thorax 2021, 76, 656–663. [Google Scholar] [CrossRef] [PubMed]

| Author and Year | Amount and Type of Patients | Nutrition Intervention | Results |
|---|---|---|---|
| Paccagnella 1994 [22] | 6 patients with CHF and mitral valve disease | 20–30 kcal/kg/d, 2 w before until 3 w after surgery | Proof of safety of nutrition in patients with severe CHF, clinical status improved |
| Tepaske 2001 [23] | 50 patients before cardiac surgery | Immune enhancing ONS, 5–10 L during 5–10 d | No adverse effects Improvements in preoperative host defense, reduced number of postoperative infections, better renal function, reduced requirement of inotropics Trend towards reduced hospital LOS |
| Aquilani 2008 [24] | 38 stable patients with CHF with sarcopenia and normal weight | Supplementation of essential amino acids for 8 w | Weight gain, increased walking capacity Improved insulin resistance (trend) Decreased lactate and pyruvate levels Improved exercise output and peak oxygen consumption |
| Rozentryt 2010 [25] | 29 CHF patients, NYHA II–IV EF < 30% | ONS: 600 kcal, 20 g protein for 6 w | Increased body weight QOL significantly improved TNF-a levels systemically reduced |
| Author and Year | Number and Type of Patients | Description of Exercise Intervention | Results |
|---|---|---|---|
| Chronic Heart Failure | |||
| Flynn, O’Connor 2009 [30,31] | 2331 stable out-patients NYHA II–IV, EF < 35% | Supervised aerobic exercise 15–35 min, 3 times/w 36 sessions, intensity: 60–70% of HR reserve | Improvement of self-reported health status Significant effect on physical and social limitations, symptoms and QOL Increased pVO2, exercise time and 6MWD |
| Sandri 2012 [32] | 60 patients with left ventricular EF < 40% | Supervised bicycle ergometry 4 × 20 min/d, for 4 w, intensity: 70% of pVO2 | Increased pVO2 and maximum work output Decreased serum levels pro-BNP |
| Lenk 2012 [33] | 24 CHF patients, NYHA IIIb | Bicycle ergometry Supervised: 3–6/d, 5–20 min, for 3 w plus, At home: 20–30 min/d for 12 w Intensity: 60% of pVO2 | Increase pVO2 Reduction in myostatin mRNA and protein levels of myostatin |
| Hoellriegel 2013 [34] | 37 CHF patients NYHA IIIb | Bicycle ergometry | Increase in skeletal muscle cross sectional area |
| Preoperative Cardiac Surgery Patients | |||
| Arthur 2000 [35] | 49 low-risk CABG | Supervised exercise + education 90 min twice/week for 8 weeks Intensity: 40–70% of functional capacity | Reduced hospital, ICU and postoperative LOS, improved QOL, greater participation in postop physical therapy |
| Herdy 2008 [36] | 56 waiting CABG | Exercise program > 5 d | Shorter time to extubation, fewer postop pulmonary complications, shorter hospital LOS |
| Rosenfeldt 2011 [37] | 17 elective cardiac surgery | Aerobic exercise and mental training 2 × 60 min/w for >2 w at 60% of max HR | No significant changes |
| Tung 2012 [38] | 35 elective cardiac surgery, NYHA I–III | Individualized exercise using a treadmill 40–60 min, 1–2 /w, >3 times at 50–60% pVO2 | Out of bed earlier, improvement in QOL |
| Critically Ill Patients | |||
| Burtin 2009 [39] | 90 critically Ill | Bedside cycling 20 min/d, 4/w Intensity: <70% of max. HR | Enhanced recovery of functional status and exercise capacity, increased muscle force Increased 6MWD and SF-36 |
| Pires-Neto 2013 [40] | 19 critically Ill | In-bed cycling 20 min | Demonstration of safety, minimal changes in vital parameters during passive cycling |
| Rahimi 2013 [41] | 3 case reports on ECMO patients | Individualized in-bed cycling | Increased lower leg strength |
| Preiser 2014 [29] | 27 critically ill | Passive in-bed cycling 2 × 30 min/d vs. 2 × 60 min/d | Less myofibrillar proteolysis No difference between groups |
| Kho 2015 [42] | 688 critically Ill | In-bed cycling Mean: 2 sessions per patient (1–4) | Cycling patients received more physical therapy interventions and were more likely to be ventilated mechanically |
| Fossat 2018 [43] | 312 critically ill adult patients | Early in-bed leg cycling 15 min/d plus electrical stimulation of the quadriceps muscles 50 min/d for 5 d/w | No difference in Medical Research Council score, ICU Mobility Score, ventilator-free days or outcomes assessed at 6 months |
| Nickels 2020 [44] | 72 mechanically ventilated | In-bed cycling 30 min/d | No significant differences in muscle atrophy strength, function or quality of life |
| Waldauf 2021 [45] | 150 mechanically ventilated < 72 h with expected ICU LOS > 7 d | Functional electrical stimulation-assisted Cycle ergometry, 90 min/d | No difference in Physical Component Summary of SF-36 at 6 months, ICU LOS, functional performance, rectus femoris cross-sectional diameter or muscle power |
| Berney 2021 [46] | 162 mechanically ventilated with expected ICU stay ≥ 4 d | Median: 5 (IQR 3–9) functional electrical stimulation-assisted cycle ergometry > 5 d/w, median duration 56 (34–63) min/d | No differences between muscle strength at hospital discharge, cognitive impairment at 6 months, or secondary outcomes measured in-hospital and at 6- and 12-month follow-up |
| Inclusion Criteria | Exclusion Criteria | Rationale for Exclusion |
|---|---|---|
| Hospital admission < 5 days prior to planned LVAD implantation | Not enough time for the patient to benefit from the intervention |
| Patients on ECMO or INTERMACS score ≤ 1 | Unable to receive proposed intervention | |
| Patients already receiving intense nutritional support, in addition to normal nutrition upon hospital admission | Confounding of results | |
| Enteral nutrition is contraindicated | Unable to receive proposed intervention | |
| Pregnant or lactating patients | Unknown effects in fetus/child | |
| Patients with clinical fulminant hepatic failure (cirrhosis Child’s class C) | Protein supplementation may be harmful in patients with severe liver disease | |
| Patients with severe clinical kidney failure (GFR < 30 mL/min) or requiring hemodialysis | Different nutritional and fluid requirements | |
| Known allergy or intolerance to study nutrients | Unable to receive proposed intervention | |
| Intracranial or spinal process affecting motor function | May not benefit from proposed intervention (i.e., different mechanism of muscle weakness) | |
| Lower extremity impairments that prevent cycling (e.g., amputation, knee/hip injury) | Unable to receive proposed cycling intervention | |
| Disabling neuropsychiatric disorders or language barriers | Unable to follow instruction or perform outcome assessments | |
| Weight > 150 kg | Exceeds maximum weight of cycle device | |
| Enrolment in an industry sponsored randomized trial within the last 30 days (co-enrolment in academic randomized trials will be considered on a case-by-case basis) | To avoid conflict of interests |
| Outcome Parameter | Description | Target |
|---|---|---|
| Safety | Occurrence of adverse effects | |
| Feasibility | Aborted cycling interventions | |
| Compliance | Percentage of patients receiving the prescribed interventions within 24 h | >80% |
| Percentage of interventions administered to the patient | >80% | |
| Efficacy | Effective separation of control and intervention group regarding caloric and protein supplementation | >10% |
| Recruitment | Successful recruitment aspired number of patients/month | >2 patients/month |
| Contamination | Avoidance of administration of ONS and cycling intervention in the control group | <5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, A.; von Dossow, V.; Heyland, D.K.; Rossaint, R.; Meybohm, P.; Fox, H.; Morshuis, M.; Elke, G.; Panholzer, B.; Haneya, A.; et al. Preoperative Nutritional Optimization and Physical Exercise for Patients Scheduled for Elective Implantation for a Left-Ventricular Assist Device—The PROPER-LVAD Study. Surgeries 2022, 3, 284-296. https://doi.org/10.3390/surgeries3040031
Hill A, von Dossow V, Heyland DK, Rossaint R, Meybohm P, Fox H, Morshuis M, Elke G, Panholzer B, Haneya A, et al. Preoperative Nutritional Optimization and Physical Exercise for Patients Scheduled for Elective Implantation for a Left-Ventricular Assist Device—The PROPER-LVAD Study. Surgeries. 2022; 3(4):284-296. https://doi.org/10.3390/surgeries3040031
Chicago/Turabian StyleHill, Aileen, Vera von Dossow, Daren K. Heyland, Rolf Rossaint, Patrick Meybohm, Henrik Fox, Michiel Morshuis, Gunnar Elke, Bernd Panholzer, Assad Haneya, and et al. 2022. "Preoperative Nutritional Optimization and Physical Exercise for Patients Scheduled for Elective Implantation for a Left-Ventricular Assist Device—The PROPER-LVAD Study" Surgeries 3, no. 4: 284-296. https://doi.org/10.3390/surgeries3040031
APA StyleHill, A., von Dossow, V., Heyland, D. K., Rossaint, R., Meybohm, P., Fox, H., Morshuis, M., Elke, G., Panholzer, B., Haneya, A., Böning, A., Niemann, B., Zayat, R., Moza, A., & Stoppe, C. (2022). Preoperative Nutritional Optimization and Physical Exercise for Patients Scheduled for Elective Implantation for a Left-Ventricular Assist Device—The PROPER-LVAD Study. Surgeries, 3(4), 284-296. https://doi.org/10.3390/surgeries3040031