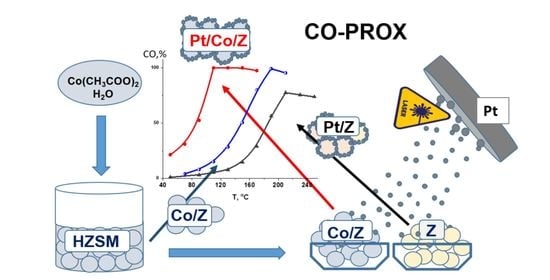
Highly Effective Pt-Co/ZSM-5 Catalysts with Low Pt Loading for Preferential CO Oxidation in H2-Rich Mixture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Tests
3. Results and Discussion
3.1. The Structure of Catalysts
3.1.1. Catalysts Morphology
3.1.2. FTIR Spectroscopy Studies
3.1.3. XPS Studies
3.2. Catalytic Performance
3.2.1. Synergistic Effect of Pt and Co in CO-PROX
3.2.2. Impact of Co/Al Ratio in 0.01Pt/Co/Z on Catalytic Performance
3.2.3. Pt content Influence on Catalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pourrahmani, H.; Yavarinasab, A.; Siavashi, M.; Matian, M.; Vanherle, J. Progress in the proton exchange membrane fuel cells (PEMFCs) water/thermal management: From theory to the current challenges and real-time fault diagnosis methods. Energy Rev. 2022, 1, 100002. [Google Scholar] [CrossRef]
- Jing, P.; Gong, X.; Liu, B.; Zhang, J. Recent advances in synergistic effect promoted catalysts for preferential oxidation of carbon monoxide. Catal. Sci. Technol. 2020, 10, 919–934. [Google Scholar] [CrossRef]
- Park, E.D.; Lee, D.; Lee, H.C. Recent progress in selective CO removal in a H2-rich stream. Catal. Tod. 2009, 139, 280–290. [Google Scholar] [CrossRef]
- Liu, J.; Hensley, A.J.R.; Giannakakis, G.; Therrien, A.J.; Sukkar, A.; Schilling, A.C.; Groden, K.; Ulumuddin, N.; Hannagan, R.T.; Ouyang, M.; et al. Developing single-site Pt catalysts for the preferential oxidation of CO: A surface science and first principles-guided approach. Appl. Catal. B 2021, 284, 119716. [Google Scholar] [CrossRef]
- Du, X.; Lang, Y.; Cao, K.; Yang, J.; Cai, Y.; Shan, B.; Chen, R. Bifunctionally faceted Pt/Ru nanoparticles for preferential oxidation of CO in H2. J. Catal. 2021, 396, 148–156. [Google Scholar] [CrossRef]
- Nguen, T.-S.; Morfin, F.; Aouine, M.; Bosselet, F.; Roussert, J.-L.; Laurent, P. Trends in the CO oxidation and PROX performances of the platinum-group metals supported on ceria. Catal. Tod. 2015, 253, 106–114. [Google Scholar] [CrossRef]
- Watanabe, M.; Uchida, H.; Ohkubo, K.; Igarashi, H. Hydrogen purification for fuel cells: Selective oxidation of carbon monoxide on Pt–Fe/zeolite catalysts. Appl. Catal. B 2003, 46, 595–600. [Google Scholar] [CrossRef]
- Marino, F.; Descorme, C.; Duprez, D. Noble metal catalysts for the preferential oxidation of carbon monoxide in the presence of hydrogen (PROX). Appl. Catal. B 2004, 54, 59–66. [Google Scholar] [CrossRef]
- Rosso, I.; Galletti, C.; Saracco, G.; Garrone, E.; Specchia, V. Development of A zeolites-supported noble-metal catalysts for CO preferential oxidation: H2 gas purification for fuel cell. Appl. Catal. B 2004, 48, 195–203. [Google Scholar] [CrossRef]
- Zlotea, C.; Oumella, Y.; Provosk, K.; Morfin, F.; Piccolo, L. Role of hydrogen absorption in supported Pd nanocatalysts during CO-PROX: Insights from operando X-ray absorption spectroscopy. Appl. Catal. B 2018, 237, 1059–1065. [Google Scholar] [CrossRef]
- Marino, F.; Descorme, C.; Duprez, D. Supported base metal catalysts for the preferential oxidation of carbon monoxide in the presence of excess hydrogen (PROX). Appl. Catal. B 2005, 58, 175–183. [Google Scholar] [CrossRef]
- Li, J.; Lei, Y.; Guo, Z.; Li, G.; Chen, X.; Zhou, F. Temperature-dependent reaction pathways of CO oxidation and the application as monolithic catalysts for Co3O4 nanorods. Appl. Catal. A 2019, 587, 117191. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Wang, Y.; Wang, T.; Gu, J.; Peng, L.; Xue, N.; Zhu, Y.; Guo, X.; Ding, W. Reduction-oxidation pretreatment enhanced catalytic performance of Co3O4/Al2O3 over CO oxidation. Appl. Surf. Sci. 2018, 453, 330–335. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic oxidation of carbon monoxide over transition metal oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Arango-Diaz, A.; Cecilia, J.A.; Marrero-Jerez, J.; Nuñez, P.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E. Freeze-dried Co3O4–CeO2 catalysts for the preferential oxidation of CO with the presence of CO2 and H2O in the feed. Ceram. Int. 2016, 46, 7462–7474. [Google Scholar] [CrossRef]
- Gawade, P.; Bayram, B.; Alexander, A.-M.C.; Ozkan, U.S. Preferential oxidation of CO (PROX) over CoOx/CeO2 in hydrogen-rich streams: Effect of cobalt loading. Appl. Catal. B 2012, 128, 21–32. [Google Scholar] [CrossRef]
- Shilina, M.I.; Rostovshchikova, T.N.; Nikolaev, S.A.; Udalova, O.V. Polynuclear Co-oxo cations in the catalytic oxidation of CO on Co-modified ZSM-5 zeolites. Mater. Chem. Phys. 2019, 223, 287–298. [Google Scholar] [CrossRef]
- Shilina, M.; Udalova, O.; Krotova, I.; Ivanin, I.; Boichenko, A. Oxidation of carbon monoxide on Co/Ce-modified ZSM-5 zeolites: Impact of mixed Oxo-Species. ChemCatChem 2020, 12, 2556–2568. [Google Scholar] [CrossRef]
- Ivanin, I.A.; Krotova, I.N.; Udalova, O.V.; Zanaveskin, K.L.; Shilina, M.I. Synergistic catalytic effect of cobalt and cerium in the preferential oxidation of carbon monoxide on modified Co/Ce/ZSM-5 Zeolites. Kin. Cat. 2021, 62, 798–811. [Google Scholar] [CrossRef]
- McClure, S.M.; Goodman, D.W. New insights into catalytic CO oxidation on Pt-group metals at elevated pressures. Chem. Phys. Lett. 2009, 469, 1–13. [Google Scholar] [CrossRef]
- Lin, J.; Wang, X.; Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Chin. J. Catal. 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Neumann, S.; Gutmann, T.; Buntkowsky, G.; Paul, S.; Thiele, G.; Sievers, H.; Blaumer, M.; Kurz, S. Insights into the reaction mechanism and particle size effects of CO oxidation over supported Pt nanoparticle catalysts. J. Catal. 2019, 377, 662–672. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhao, Y.; Chen, M.; Wan, H. Effect of dispersion on catalytic performance of supported Pt catalysts for CO oxidation. Chin. J. Catal. 2012, 33, 1901–1905. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Li, L.; Pan, X.; Wang, X.; Zhang, T. Local structure of Pt species dictates remarkable performance on Pt/Al2O3 for preferential oxidation of CO in H2. Appl. Catal. B 2021, 282, 119588. [Google Scholar] [CrossRef]
- Pakharukova, V.P.; Pakharukov, I.Y.; Bukhtiyarov, V.I.; Parmon, V.N. Alumina-supported platinum catalysts: Local atomic structure and catalytic activity for complete methane oxidation. Appl. Catal. A 2014, 486, 12–18. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Hu, Y.; Tian, M. Catalytic oxidation of CO over Pt/TiO2 with low Pt loading: The effect of H2O and SO2. Appl. Catal. A 2021, 622, 118218. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Li, L.; Lin, Q.; Wei, H.; Liu, J.; Zhang, T. Ferric oxide-supported Pt subnano clusters for preferential oxidation of CO in H2-rich gas at room temperature. ACS Catal. 2014, 4, 2113–2117. [Google Scholar] [CrossRef]
- Lang, R.; Du, X.; Huang, Y.; Jiang, X.; Zhang, Q.; Guo, Y.; Liu, K.; Qiao, B.; Wang, A.; Zhang, T. Single-atom catalysts based on the metal−oxide interaction. Chem. Rev. 2020, 120, 11986–12043. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Lee, S.; Yang, S.; Liu, J.; Giannakakis, G.; Li, M.; Ouyang, M.; Wang, D.; Sykes, E.C.H.; et al. High-loading single Pt atom sites [Pt-O (OH)x] catalyze the CO PROX reaction with high activity and selectivity at mild conditions. Sci. Adv. 2020, 6, eaba3809. [Google Scholar] [CrossRef]
- Niu, T.; Zhao, W.W.; Liu, G.L.; Cao, A.; Zhang, L.H.; Liu, Y. The graphene-meso-macro-porous SiO2 supported Pt-Ni alloy nanocatalyst for preferential oxidation of CO in H2-rich gases. Int. J. Hydrog. Energy 2014, 39, 18929–18939. [Google Scholar] [CrossRef]
- Teschner, D.; Wootscha, A.; Paál, Z. Preferential CO oxidation in hydrogen (PROX) on unsupported PtSn catalyst. Appl. Catal. A 2012, 411–412, 31–34. [Google Scholar] [CrossRef]
- Tripathi, A.; Hareesh, C.; Sinthika, S.; Andersson, G.; Thapa, R. CO oxidation on Pt based binary and ternary alloy nanocatalysts: Reaction pathways and electronic descriptor. Appl. Surf. Sci. 2020, 528, 146964. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Zhang, N.; Zheng, J.; Zheng, Y.; Li, Y.; Zhong, C.-J.; Chen, B.H. Construction of ultrafine and stable PtFe nano-alloy with ultra-low Pt loading for complete removal of CO in PROX at room temperature. Appl. Catal. B 2016, 180, 237–245. [Google Scholar] [CrossRef]
- Potemkin, D.I.; Filatov, E.Y.; Zadesenets, A.V.; Gorlova, A.M.; Nikitina, N.A.; Pichugina, D.A. A comparative study of CO preferential oxidation over Pt and Pt0.5Co0.5 nanoparticles: Kinetic study and quantum-chemical calculations. Mater. Lett. 2020, 260, 126915. [Google Scholar] [CrossRef]
- Mohamed, Z.; Dasireddy, V.D.B.C.; Singh, S.; Fiedrich, H.B. The preferential oxidation of CO in hydrogen rich streams over platinum doped nickel oxide catalysts. Appl. Catal. B 2016, 180, 687–697. [Google Scholar] [CrossRef]
- Wang, C.X.; Zhang, L.; Liu, Y. Aluminumphosphate molecular sieves supported Pt–Co catalysts for the preferential oxidation of CO in H2-rich gases. Appl. Catal. B 2013, 136–137, 48–55. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Tu, S.-T.; Yan, J.; Wang, Z. Catalytic performance and characterization of Al2O3-supported Pt–Co catalyst coatings for preferential CO oxidation in a micro-reactor. Appl. Catal. A 2010, 387, 215–223. [Google Scholar] [CrossRef]
- Cai, J.; Liu, Z.; Cao, K.; Lang, Y.; Chu, S.; Shan, B.; Chen, R. Highly dispersed Pt studded on CoOx nanoclusters for CO preferential oxidation in H2. J. Mater. Chem. A 2020, 8, 10180–10187. [Google Scholar] [CrossRef]
- Potemkin, D.I.; Filatov, E.Y.; Zadesenets, A.V.; Snytnikov, P.V.; Shubin, Y.V.; Sobyanin, V.A. Preferential CO oxidation over bimetallic Pt–Co catalysts prepared via double complex salt decomposition. Chem. Eng. J. 2012, 207–208, 683–689. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Lin, H.; Yuan, Y. Carbon nanotube-supported Pt-Co bimetallic catalysts for preferential oxidation of CO in a H2-rich stream with CO2 and H2O vapor. J. Power Sources 2012, 202, 200–208. [Google Scholar] [CrossRef]
- Nunez, N.E.; Bideberripe, H.P.; Mizrahi, M.; Ramallo-López, J.M.; Casella, M.L.; Siri, G.J. CO selective oxidation using Co-promoted Pt/γ-Al2O3 catalysts. Int. J. Hydrog. Energy 2016, 41, 19005–19013. [Google Scholar] [CrossRef]
- Freund, H.-J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064. [Google Scholar] [CrossRef] [PubMed]
- Paz, D.S.; Damyanov, S.; Borges, L.R.; Santos, J.B.O.; Bueno, J.M.C. Identifying the adsorbed active intermediates on Pt surface and promotion of activity through the redox CeO2 in preferential oxidation of CO in H2. Appl. Catal. A 2017, 548, 164–178. [Google Scholar] [CrossRef]
- Teschner, D.; Wootsch, A.; Pozdnyakova-Tellinger, O.; Kröhnert, J.; Vass, E.M.; Hävecker, M.; Zafeiratos, S.; Schnörch, P.; Jentoft, P.C.; Knop-Gericke, A.; et al. Partial pressure dependent in situ spectroscopic study on the preferential CO oxidation in hydrogen (PROX) over Pt/ceria catalysts. J. Catal. 2007, 249, 318–327. [Google Scholar] [CrossRef]
- Komatsu, T.; Tamura, A. Pt3Co and PtCu intermetallic compounds: Promising catalysts for preferential oxidation of CO in excess hydrogen. J. Catal. 2008, 258, 306–314. [Google Scholar] [CrossRef]
- Lopez, A.; Navascues, N.; Mallada, R.; Irusta, S. Pt-CoOx nanoparticles supported on ETS-10 for preferential oxidation of CO reaction. Appl. Catal. A 2016, 528, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Cuenya, B.R. Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects. Thin Solid Films 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Uzio, D.; Berhault, G. Factors governing the catalytic reactivity of metallic nanoparticles. Catal. Rev. 2010, 52, 106–131. [Google Scholar] [CrossRef]
- Lokteva, E.S.; Golubina, E.V. Metal-support interactions in the design of heterogeneous catalysts for redox processes. Pure Appl. Chem. 2019, 91, 609. [Google Scholar] [CrossRef]
- Xu, J.; Xu, X.-C.; Ouyang, L.; Yang, X.-J.; Mao, W.; Su, J.; Han, Y.-F. Mechanistic study of preferential CO oxidation on a Pt/NaY zeolite catalyst. J. Catal. 2012, 287, 114–123. [Google Scholar] [CrossRef]
- Sebastian, V.; Irusta, S.; Mallada, R.; Santamaría, J. Microreactors with Pt/zeolite catalytic films for the selective oxidation of CO in simulated reformer streams. Catal. Tod. 2009, 147S, S10–S16. [Google Scholar] [CrossRef]
- Sebastian, V.; Irusta, S.; Mallada, R.; Santamaría, J. Selective oxidation of CO in the presence of H2, CO2 and H2O on different zeolite-supported Pt catalysts. Appl. Catal. A 2009, 366, 242–251. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Wu, J.; Sun, D.; Li, Q. Enhancing water resistance of Pt nanoparticles by tailoring microenvironment of hollow ZSM-5 for efficient benzene oxidation. Chem. Eng. J. 2023, 451, 138351. [Google Scholar] [CrossRef]
- Guo, J.; Ding, C.; Ma, Z.; Ma, L.; Wang, J.; Shangguan, J.; Yuan, Q.; Zhao, M.; Li, Y.; Wang, M.; et al. Highly dispersed and stable Pt clusters encapsulated within ZSM-5 with aid of sodium ion for partial oxidation of methane. Fuel 2021, 289, 119839. [Google Scholar] [CrossRef]
- Kong, F.; Li, G.; Wang, J.; Shi, Y.; Zhou, R. Promoting effect of acid sites in hierarchical porous Pt/ZSM-5 catalysts for low-temperature removal of VOCs. Appl. Surf. Sci. 2022, 606, 154888. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, D.; Deng, W.; Guo, L. Different morphological ZSM-5 zeolites supported Pt catalysts for toluene catalytic combustion. Chem. Phys. Impact. 2022, 5, 100134. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Alotaibi, M.T.; El-Bahy, S.M. CO oxidation and 4-nitrophenol reduction over ceria-promoted platinum nanoparticles impregnated with ZSM-5 zeolite. J. Rare Earths 2022, 40, 1247–1254. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Shi, Y.; Zhou, R. Synergistic effect of Pt nanoparticles and micro-mesoporous ZSM-5 in VOCs low-temperature removal. J. Environ. Sci. 2021, 107, 87–97. [Google Scholar] [CrossRef]
- Babucci, M.; Guntida, A.; Gates, B.C. Atomically dispersed metals on well-defined supports including zeolites and metal−organic frameworks: Structure, bonding, reactivity, and catalysis. Chem. Rev. 2020, 120, 11956–11985. [Google Scholar] [CrossRef]
- Michalak, W.D.; Krier, J.M.; Alayoglu, S.; Shin, J.-Y.; An, K.; Komvopoulos, K.; Liu, Z.; Somorjai, G.A. CO oxidation on PtSn nanoparticle catalysts occurs at the interface of Pt and Sn oxide domains formed under reaction conditions. J. Catal. 2014, 312, 17–25. [Google Scholar] [CrossRef]
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Müller, A.M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Lokteva, E.S.; Shilina, M.I.; Golubina, E.V.; Maslakov, K.I.; Krotova, I.N.; Bryzhin, A.A.; Tarkhanova, I.G.; Udalova, O.V.; Kozhevin, V.M.; et al. Laser electrodispersion of metals for the synthesis of nanostructured catalysts: Achievements and prospects. Russ. J. Phys. Chem. A 2021, 95, 451–474. [Google Scholar] [CrossRef]
- Bryzhin, A.A.; Golubina, E.V.; Maslakov, K.I.; Lokteva, E.S.; Tarkhanova, I.G.; Gurevich, S.A.; Yavsin, D.A.; Rostovshchikova, T.N. Bimetallic nanostructured catalysts prepared by laser electrodispersion: Structure and activity in redox reactions. ChemCatChem 2020, 12, 4396–4405. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Nikolaev, S.A.; Krotova, I.N.; Maslakov, K.I.; Udalova, O.V.; Gurevich, S.A.; Yavsin, D.A.; Shilina, M.I. ZSM-5 and BEA zeolites modified with Pd nanoparticles by laser electrodispersion. The structure and catalytic activity in CO and CH4 oxidation. Russ. Chem. Bull. 2022, 71, 1179–1193. [Google Scholar] [CrossRef]
- Golubina, E.V.; Rostovshchikova, T.N.; Lokteva, E.S.; Maslakov, K.I.; Nikolaev, S.A.; Shilina, M.I.; Gurevich, S.A.; Kozhevin, V.M.; Yavsin, D.A.; Slavinskay, E.M. Role of surface coverage of alumina with Pt nanoparticles deposited by laser electrodispersion in catalytic CO oxidation. Appl. Surf. Sci. 2021, 536, 147656. [Google Scholar] [CrossRef]
- Rostovshchikova, T.N.; Shilina, M.I.; Gurevich, S.A.; Yavsin, D.A.; Veselov, G.B.; Vedyagin, A.A. New approaches to the synthesis of ultralow-palladium automotive emission control catalysts. Dokl. Phys. Chem. 2022, 506, 123–130. [Google Scholar] [CrossRef]
- Chupin, C.; van Veen, A.C.; Konduru, M.; Deprés, J.; Mirodatos, C. Identity and location of active species for NO reduction by CH4 over Co-ZSM-5. J. Catal. 2006, 241, 103–114. [Google Scholar] [CrossRef]
- Coudurier, G.; Naccache, C.; Vedrine, J.C. Uses of I.R. spectroscopy in identifying ZSM zeolite structure. J. Chem. Soc. Chem. Commun. 1982, 24, 1413–1415. [Google Scholar] [CrossRef]
- Tang, C.-W.; Wang, C.-B.; Chien, S.-H. Characterization of cobalt oxides studied by FT-IR, Raman, TPR and TG-MS. Termochimica Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Eurov, D.A.; Rostovshchikova, T.N.; Shilina, M.I.; Kirilenko, D.A.; Tomkovich, M.V.; Yagovkina, M.A.; Udalova, O.V.; Kaplin, I.Y.; Ivanin, I.A.; Kurdyukov, D.A. Cobalt oxide decorated porous silica particles: Structure and activity relationship in the catalytic oxidation of carbon monoxide. Appl. Surf. Sci. 2022, 579, 152121. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Yu, H.; Wang, X. The functions of Pt located at different positions of HZSM-5 in H2-SCR. Chem. Eng. J. 2019, 355, 470–477. [Google Scholar] [CrossRef]
- Gao, M.; Gong, Z.; Weng, X.; Shang, W.; Chai, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Methane combustion over palladium catalyst within the confined space of MFI zeolite. Chin. J. Catal. 2021, 42, 1689–1699. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Lukashuk, L.; Fottinger, K.; Kolar, E.; Rameshan, C.; Teschner, D.; Havecker, M.; Knop-Gericke, A.; Yigit, N.; Li, H.; McDermott, E.; et al. Operando XAS and NAP-XPS studies of preferential CO oxidation on Co3O4 and CeO2-Co3O4 catalysts. J. Catal. 2016, 344, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Morfin, F.; Nguyen, T.-S.; Rousset, J.-L.; Piccolo, L. Synergy between hydrogen and ceria in Pt-catalyzed CO oxidation: An investigation on Pt–CeO2 catalysts synthesized by solution combustion. Appl. Catal. B Environ. 2016, 197, 2–13. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, M.; Cai, D.; Tan, K.B.; Zhan, G. Fabrication of Pt/Co3O4 nanocatalysts based on pollen template for low-temperature CO oxidation. Cat. Commun. 2023, 174, 106597. [Google Scholar] [CrossRef]
- Li, C.; Ke, C.; Han, R.; Fan, G.; Yang, L.; Li, F. The remarkable promotion of in situ formed Pt-cobalt oxide interfacial sites on the carbonyl reduction to allylic alcohols. Molecr. Catal. 2018, 455, 78–87. [Google Scholar] [CrossRef]









| Sample | Si/Al | Pt, wt.% | Co, wt.% | Co/Al 1 |
|---|---|---|---|---|
| 0.01Pt/Z-15 | 15 | 0.01 | - | - |
| 0.05Pt/Z-15 | 15 | 0.05 | - | - |
| 0.01Pt /Z-28 | 28 | 0.01 | - | - |
| 0.05Pt /Z-28 | 28 | 0.05 | - | |
| 4.5Co/Z-15 | 15 | - | 4.5 | 0.75 |
| 4.5Co/Z-28 | 28 | - | 4.5 | 1.4 |
| 2.5Co/0.01Pt/Z-15 | 15 | 0.01 | 2.5 | 0.42 |
| 0.01Pt/2.5Co/Z-15 | 15 | 0.01 | 2.5 | 0.42 |
| 0.01Pt/4.5Co/Z-15 | 15 | 0.01 | 4.5 | 0.75 |
| 0.05Pt/4.5Co/Z-15 | 15 | 0.05 | 4.5 | 0.75 |
| 0.01Pt/2.5Co/Z-28 | 28 | 0.01 | 2.5 | 0.75 |
| 4.5Co/0.01Pt/Z-28 | 28 | 0.01 | 4.5 | 1.4 |
| 0.01 Pt/4.5Co/Z-28 | 28 | 0.01 | 4.5 | 1.4 |
| 0.05Pt/2.5Co/Z-28 | 28 | 0.05 | 2.5 | 0.75 |
| 0.01Pt/2,5Co/Z-40 | 40 | 0.01 | 2.5 | 1.1 |
| 0.01Pt/4.5Co/Z-40 | 40 | 0.01 | 4.5 | 1.8 |
| Sample | SBET, m2/g | SV-t, m2/g | Vpore, cm3/g | |
|---|---|---|---|---|
| Micropore | External | |||
| Z-15 | 444 | 425 | 19 | 0.18 |
| 4.5Co/Z-15 | 389 | 368 | 21 | 0.15 |
| 0.01Pt/Z-15 | 428 | 408 | 20 | 0.17 |
| 0.01Pt/4.5Co/Z-15 | 350 | 320 | 30 | 0.14 |
| 0.05Pt/4.5Co/Z-15 | 388 | 370 | 18 | 0.15 |
| Z-28 | 462 | 446 | 16 | 0.18 |
| 2.5Co/Z-28 | 436 | 419 | 17 | 0.15 |
| 4.5Co/Z-28 | 425 | 406 | 19 | 0.15 |
| 0.05Pt/Z-28 | 490 | 474 | 16 | 0.19 |
| 0.05Pt/2.5Co/Z-28 | 446 | 419 | 27 | 0.17 |
| Sample | Co2p3/2 | Pt4f7/2 | |||
|---|---|---|---|---|---|
| Co(III) | Co(II) | Pt0 | Ptδ+ (PtO) | Ptn+ (PtOxAl) | |
| Pt/Z | – | – | 71.4–71.6 | 72.4–72.6 | 74.0–74.5 |
| Co/Z | 779.8–780.4 | 782.0–782.6 | – | – | – |
| Pt/Co/Z | 780.0–780.4 | 781.9–783.0 | 71.6–71.7 | 72.6–72.7 | 74.2–74.7 |
| Sample | Co, at.% | Co/(Si + Al) | Pt/(Si + Al) | Pt, at.% | ||||
|---|---|---|---|---|---|---|---|---|
| Co(III) | Co(II) | Pt0 | Ptδ+ | Pt2+ | ||||
| 0.01Pt/Z-15 | - | - | - | 0.05 | 36 | 21 | 43 | |
| 0.01Pt/Z-28 | - | - | - | 0.11 | 35 | 49 | 16 | |
| 0.05Pt/Z-28 | - | - | - | 0.22 | 61 | 24 | 15 | |
| 4.5Co/Z-15 | 46 | 54 | 0.12 | - | - | - | - | |
| 4.5Co/Z-28 | 39 | 61 | 0.17 | - | - | - | - | |
| 0.01Pt/4.5Co/Z-15 | initial | 45 | 55 | 0.20 | 0.15 | 24 | 45 | 31 |
| spent | 54 | 46 | 0.29 | 0.13 | 46 | 34 | 20 | |
| 0.01 Pt/4.5Co/Z-28 | 16 | 84 | 0.09 | 0.10 | 20 | 50 | 30 | |
| 4.5Co/0.01 Pt/Z-28 | 61 | 39 | 0.12 | 0.01 | 12 | 48 | 40 | |
| 0.05Pt/2.5Co/Z-28 | initial | 47 | 53 | 0.11 | 0.28 | 57 | 20 | 23 |
| spent | 75 | 25 | 0.32 | 0.24 | 77 | 8 | 15 | |
| Catalyst | T50, °C | COmax,% | ΔT100 (Tmax), °C |
|---|---|---|---|
| 0.01Pt/2.5Co/Z-15 | 130 | 99 | 170 |
| 2.5Co/0.01Pt/Z-15 | 108 | 100 | 130–150 |
| 0.01Pt/4.5Co/Z-15 | 90 | 100 | 110–150 |
| 0.05Pt/4.5Co/Z-15 | <50 | 100 | 50–150 |
| 0.01Pt/2.5Co/Z-28 | 97 | 100 | 130–170 |
| 0.01 Pt/4.5Co/Z-28 | 86 | 100 | 110–130 |
| 4.5Co/0.01Pt/Z-28 | 104 | 100 | 130 |
| 0.05Pt/2.5Co/Z-28 | <50 | 100 | 60–150 |
| 0.01Pt/2.5Co/Z-40 | 100 | 100 | 130–150 |
| 0.01Pt/4.5Co/Z-40 | 95 | 100 | 130 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilina, M.; Krotova, I.; Nikolaev, S.; Gurevich, S.; Yavsin, D.; Udalova, O.; Rostovshchikova, T. Highly Effective Pt-Co/ZSM-5 Catalysts with Low Pt Loading for Preferential CO Oxidation in H2-Rich Mixture. Hydrogen 2023, 4, 154-173. https://doi.org/10.3390/hydrogen4010011
Shilina M, Krotova I, Nikolaev S, Gurevich S, Yavsin D, Udalova O, Rostovshchikova T. Highly Effective Pt-Co/ZSM-5 Catalysts with Low Pt Loading for Preferential CO Oxidation in H2-Rich Mixture. Hydrogen. 2023; 4(1):154-173. https://doi.org/10.3390/hydrogen4010011
Chicago/Turabian StyleShilina, Marina, Irina Krotova, Sergey Nikolaev, Sergey Gurevich, Denis Yavsin, Olga Udalova, and Tatiana Rostovshchikova. 2023. "Highly Effective Pt-Co/ZSM-5 Catalysts with Low Pt Loading for Preferential CO Oxidation in H2-Rich Mixture" Hydrogen 4, no. 1: 154-173. https://doi.org/10.3390/hydrogen4010011
APA StyleShilina, M., Krotova, I., Nikolaev, S., Gurevich, S., Yavsin, D., Udalova, O., & Rostovshchikova, T. (2023). Highly Effective Pt-Co/ZSM-5 Catalysts with Low Pt Loading for Preferential CO Oxidation in H2-Rich Mixture. Hydrogen, 4(1), 154-173. https://doi.org/10.3390/hydrogen4010011