The Role of T Cells in Systemic Sclerosis: An Update
Abstract
:1. Introduction
2. Antigenic Activation of T Cells in Systemic Sclerosis
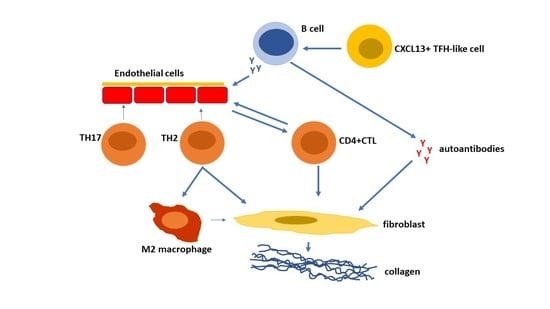
3. T Cell Subsets and Function in Systemic Sclerosis
4. Therapeutic Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Van Caam, A.; Vonk, M.; Van Den Hoogen, F.; Van Lent, P.; Van Der Kraan, P. Unraveling SSc Pathophysiology; The Myofibroblast. Front. Immunol. 2018, 9, 2452. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y. The Pathogenesis of Systemic Sclerosis: An Understanding Based on a Common Pathologic Cascade across Multiple Organs and Additional Organ-Specific Pathologies. J. Clin. Med. 2020, 9, 2687. [Google Scholar] [CrossRef] [PubMed]
- Truchetet, M.E.; Brembilla, N.C.; Chizzolini, C. Current Concepts on the Pathogenesis of Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Liaskos, C.; Marou, E.; Simopoulou, T.; Barmakoudi, M.; Efthymiou, G.; Scheper, T.; Meyer, W.; Bogdanos, D.P.; Sakkas, L.I. Disease-related autoantibody profile in patients with systemic sclerosis. Autoimmunity 2017, 50, 414–421. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Bogdanos, D.P. Systemic sclerosis: New evidence re-enforces the role of B cells. Autoimmun. Rev. 2016, 15, 155–161. [Google Scholar] [CrossRef]
- Fukasawa, T.; Yoshizaki, A.; Ebata, S.; Yoshizaki-Ogawa, A.; Asano, Y.; Enomoto, A.; Miyagawa, K.; Kazoe, Y.; Mawatari, K.; Kitamori, T.; et al. Single-cell-level protein analysis revealing the roles of autoantigen-reactive B lymphocytes in autoimmune disease and the murine model. Elife 2021, 10, e67209. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Chikanza, I.C.; Platsoucas, C.D. Mechanisms of Disease: The role of immune cells in the pathogenesis of systemic sclerosis. Nat. Clin. Pract. Rheumatol. 2006, 2, 679–685. [Google Scholar] [CrossRef]
- Sgonc, R.; Gruschwitz, M.S.; Dietrich, H.; Recheis, H.; Gershwin, M.E.; Wick, G. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J. Clin. Investig. 1996, 98, 785–792. [Google Scholar] [CrossRef]
- Frasca, L.; Lande, R. Toll-like receptors in mediating pathogenesis in systemic sclerosis. Clin. Exp. Immunol. 2020, 201, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Skaug, B.; Khanna, D.; Swindell, W.R.; Hinchcliff, M.E.; Frech, T.M.; Steen, V.D.; Hant, F.N.; Gordon, J.K.; Shah, A.A.; Zhu, L.; et al. Global skin gene expression analysis of early diffuse cutaneous systemic sclerosis shows a prominent innate and adaptive inflammatory profile. Ann. Rheum. Dis. 2020, 79, 379–386. [Google Scholar] [CrossRef]
- Henderson, J.; Bhattacharyya, S.; Varga, J.; O’Reilly, S. Targeting TLRs and the inflammasome in systemic sclerosis. Pharmacol. Ther. 2018, 192, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Skaug, B.; Bi, X.; Mills, T.; Salazar, G.; Zhou, X.; Reveille, J.; Agarwal, S.K.; Blackburn, M.R.; Mayes, M.D.; et al. Interferon regulatory factor 7 (IRF7) represents a link between inflammation and fibrosis in the pathogenesis of systemic sclerosis. Ann. Rheum. Dis. 2019, 78, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Kafaja, S.; Valera, I.; Divekar, A.A.; Saggar, R.; Abtin, F.; Furst, D.E.; Khanna, D.; Singh, R.R. pDCs in lung and skin fibrosis in a bleomycin-induced model and patients with systemic sclerosis. JCI Insight 2018, 3, e98380. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Peck, A.; Santer, D.; Patole, P.; Schwartz, S.M.; Molitor, J.A.; Arnett, F.C.; Elkon, K.B. Induction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: Association of higher interferon-alpha activity with lung fibrosis. Arthritis Rheum. 2008, 58, 2163–2173. [Google Scholar] [CrossRef]
- Van Bon, L.; Affandi, A.J.; Broen, J.; Christmann, R.B.; Marijnissen, R.J.; Stawski, L.; Farina, G.A.; Stifano, G.; Mathes, A.L.; Cossu, M.; et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 2014, 370, 433–443. [Google Scholar] [CrossRef]
- Muskardin, T.L.W.; Niewold, T.B. Type I interferon in rheumatic diseases. Nat. Rev. Rheumatol. 2018, 14, 214–228. [Google Scholar] [CrossRef]
- Lande, R.; Mennella, A.; Palazzo, R.; Pietraforte, I.; Stefanantoni, K.; Iannace, N.; Butera, A.; Boirivant, M.; Pica, R.; Conrad, C.; et al. Anti-CXCL4 Antibody Reactivity Is Present in Systemic Sclerosis (SSc) and Correlates with the SSc Type I Interferon Signature. Int. J. Mol. Sci. 2020, 21, 5102. [Google Scholar] [CrossRef]
- Lande, R.; Lee, E.Y.; Palazzo, R.; Marinari, B.; Pietraforte, I.; Santos, G.S.; Mattenberger, Y.; Spadaro, F.; Stefanantoni, K.; Iannace, N.; et al. CXCL4 assembles DNA into liquid crystalline complexes to amplify TLR9-mediated interferon-alpha production in systemic sclerosis. Nat. Commun. 2019, 10, 1731. [Google Scholar] [CrossRef]
- Ntelis, K.; Solomou, E.E.; Sakkas, L.; Liossis, S.N.; Daoussis, D. The role of platelets in autoimmunity, vasculopathy, and fibrosis: Implications for systemic sclerosis. Semin. Arthritis Rheum. 2017, 47, 409–417. [Google Scholar] [CrossRef]
- Maehara, T.; Kaneko, N.; Perugino, C.A.; Mattoo, H.; Kers, J.; Allard-Chamard, H.; Mahajan, V.S.; Liu, H.; Murphy, S.J.; Ghebremichael, M.; et al. Cytotoxic CD4+ T lymphocytes may induce endothelial cell apoptosis in systemic sclerosis. J. Clin. Investig. 2020, 130, 2451–2464. [Google Scholar] [CrossRef] [PubMed]
- Paleja, B.; Low, A.H.L.; Kumar, P.; Saidin, S.; Lajam, A.; Nur Hazirah, S.; Chua, C.; Li Yun, L.; Albani, S. Systemic Sclerosis Perturbs the Architecture of the Immunome. Front. Immunol. 2020, 11, 1602. [Google Scholar] [CrossRef] [PubMed]
- Fleury, M.; Belkina, A.C.; Proctor, E.A.; Zammitti, C.; Simms, R.W.; Lauffenburger, D.A.; Snyder-Cappione, J.E.; Lafyatis, R.; Dooms, H. Increased Expression and Modulated Regulatory Activity of Coinhibitory Receptors PD-1, TIGIT, and TIM-3 in Lymphocytes From Patients With Systemic Sclerosis. Arthritis Rheumatol. 2018, 70, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, A.; Lutz, K.; Winheim, E.; Krug, A.B. What Makes a pDC: Recent Advances in Understanding Plasmacytoid DC Development and Heterogeneity. Front. Immunol. 2019, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.N.; Jiang, W.; Bhamidipati, K.; Millican, M.; Macaubas, C.; Hung, S.C.; Mellins, E.D. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front. Immunol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Xu, B.; Artlett, C.M.; Lu, S.; Jimenez, S.A.; Platsoucas, C.D. Oligoclonal T cell expansion in the skin of patients with systemic sclerosis. J. Immunol. 2002, 168, 3649–3659. [Google Scholar] [CrossRef]
- De Palma, R.; Del Galdo, F.; Lupoli, S.; Altucci, P.; Abbate, G.; Valentini, G. Peripheral T lymphocytes from patients with early systemic sclerosis co-cultured with autologous fibroblasts undergo an oligoclonal expansion similar to that occurring in the skin. Clin. Exp. Immunol. 2006, 144, 169–176. [Google Scholar] [CrossRef]
- Servaas, N.H.; Zaaraoui-Boutahar, F.; Wichers, C.G.K.; Ottria, A.; Chouri, E.; Affandi, A.J.; Silva-Cardoso, S.; Van Der Kroef, M.; Carvalheiro, T.; Van Wijk, F.; et al. Longitudinal analysis of T-cell receptor repertoires reveals persistence of antigen-driven CD4(+) and CD8(+) T-cell clusters in systemic sclerosis. J. Autoimmun. 2021, 117, 102574. [Google Scholar] [CrossRef]
- Fox, D.A.; Lundy, S.K.; Whitfield, M.L.; Berrocal, V.; Campbell, P.; Rasmussen, S.; Ohara, R.; Stinson, A.; Gurrea-Rubio, M.; Wiewiora, E.; et al. Lymphocyte subset abnormalities in early diffuse cutaneous systemic sclerosis. Arthritis Res. Ther. 2021, 23, 10. [Google Scholar] [CrossRef]
- Lunardi, C.; Dolcino, M.; Peterlana, D.; Bason, C.; Navone, R.; Tamassia, N.; Beri, R.; Corrocher, R.; Puccetti, A. Antibodies against human cytomegalovirus in the pathogenesis of systemic sclerosis: A gene array approach. PLoS Med. 2006, 3, e2. [Google Scholar] [CrossRef] [Green Version]
- Scaletti, C.; Vultaggio, A.; Bonifacio, S.; Emmi, L.; Torricelli, F.; Maggi, E.; Romagnani, S.; Piccinni, M.P. Th2-oriented profile of male offspring T cells present in women with systemic sclerosis and reactive with maternal major histocompatibility complex antigens. Arthritis Rheum. 2002, 46, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Tan, J.; Chen, W.; Du, Q.; Xie, B.; Wang, N.; Zhu, H.; Wang, K. The Fibrosis and Immunological Features of Hypochlorous Acid Induced Mouse Model of Systemic Sclerosis. Front. Immunol. 2019, 10, 1861. [Google Scholar] [CrossRef]
- Maria, A.T.J.; Toupet, K.; Maumus, M.; Rozier, P.; Vozenin, M.C.; Le Quellec, A.; Jorgensen, C.; Noel, D.; Guilpain, P. Fibrosis Development in HOCl-Induced Systemic Sclerosis: A Multistage Process Hampered by Mesenchymal Stem Cells. Front. Immunol. 2018, 9, 2571. [Google Scholar] [CrossRef] [PubMed]
- Prescott, R.J.; Freemont, A.J.; Jones, C.J.; Hoyland, J.; Fielding, P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J. Pathol. 1992, 166, 255–263. [Google Scholar] [CrossRef]
- Bosello, S.; Angelucci, C.; Lama, G.; Alivernini, S.; Proietti, G.; Tolusso, B.; Sica, G.; Gremese, E.; Ferraccioli, G. Characterization of inflammatory cell infiltrate of scleroderma skin: B cells and skin score progression. Arthritis Res. Ther. 2018, 20, 75. [Google Scholar] [CrossRef]
- Kalogerou, A.; Gelou, E.; Mountantonakis, S.; Settas, L.; Zafiriou, E.; Sakkas, L. Early T cell activation in the skin from patients with systemic sclerosis. Ann. Rheum. Dis. 2005, 64, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Phadungsaksawasdi, P.; Kurihara, K.; Fujiyama, T.; Honda, T. Pathophysiology of Skin Resident Memory T Cells. Front. Immunol. 2020, 11, 618897. [Google Scholar] [CrossRef]
- Samat, A.A.K.; Van der Geest, J.; Vastert, S.J.; van Loosdregt, J.; van Wijk, F. Tissue-Resident Memory T Cells in Chronic Inflammation-Local Cells with Systemic Effects? Cells 2021, 10, 409. [Google Scholar] [CrossRef]
- O’Reilly, S.; Hugle, T.; Van Laar, J.M. T cells in systemic sclerosis: A reappraisal. Rheumatology 2012, 51, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, L.I.; Platsoucas, C.D. Is systemic sclerosis an antigen-driven T cell disease? Arthritis Rheum. 2004, 50, 1721–1733. [Google Scholar] [CrossRef]
- Li, G.; Larregina, A.T.; Domsic, R.T.; Stolz, D.B.; Medsger, T.A., Jr.; Lafyatis, R.; Fuschiotti, P. Skin-Resident Effector Memory CD8(+)CD28(−) T Cells Exhibit a Profibrotic Phenotype in Patients with Systemic Sclerosis. J. Investig. Dermatol. 2017, 137, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Almanzar, G.; Schmalzing, M.; Klein, M.; Hilligardt, D.; Morris, P.; Hofner, K.; Hajj, N.E.; Kneitz, H.; Wild, V.; Rosenwald, A.; et al. Memory CD4+ T cells lacking expression of CCR7 promote pro-inflammatory cytokine production in patients with diffuse cutaneous systemic sclerosis. Eur. J. Dermatol. EJD 2019, 29, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Chizzolini, C.; Parel, Y.; De Luca, C.; Tyndall, A.; Akesson, A.; Scheja, A.; Dayer, J.M. Systemic sclerosis Th2 cells inhibit collagen production by dermal fibroblasts via membrane-associated tumor necrosis factor alpha. Arthritis Rheum. 2003, 48, 2593–2604. [Google Scholar] [CrossRef] [PubMed]
- Parel, Y.; Aurrand-Lions, M.; Scheja, A.; Dayer, J.M.; Roosnek, E.; Chizzolini, C. Presence of CD4+CD8+ double-positive T cells with very high interleukin-4 production potential in lesional skin of patients with systemic sclerosis. Arthritis Rheum. 2007, 56, 3459–3467. [Google Scholar] [CrossRef]
- Kanno, Y.; Shu, E.; Niwa, H.; Kanoh, H.; Seishima, M. Alternatively activated macrophages are associated with the alpha2AP production that occurs with the development of dermal fibrosis: The role of alternatively activated macrophages on the development of fibrosis. Arthritis Res. Ther. 2020, 22, 76. [Google Scholar] [CrossRef] [PubMed]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef]
- Higashioka, K.; Kikushige, Y.; Ayano, M.; Kimoto, Y.; Mitoma, H.; Kikukawa, M.; Akahoshi, M.; Arinobu, Y.; Horiuchi, T.; Akashi, K.; et al. Generation of a novel CD30(+) B cell subset producing GM-CSF and its possible link to the pathogenesis of systemic sclerosis. Clin. Exp. Immunol. 2020, 201, 233–243. [Google Scholar] [CrossRef]
- Binai, N.; O’Reilly, S.; Griffiths, B.; Van Laar, J.M.; Hugle, T. Differentiation potential of CD14+ monocytes into myofibroblasts in patients with systemic sclerosis. PLoS ONE 2012, 7, e33508. [Google Scholar]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef]
- Yaseen, B.; Lopez, H.; Taki, Z.; Zafar, S.; Rosario, H.; Abdi, B.A.; Vigneswaran, S.; Xing, F.; Arumalla, N.; Black, S.; et al. Interleukin-31 promotes pathogenic mechanisms underlying skin and lung fibrosis in scleroderma. Rheumatology 2020, 59, 2625–2636. [Google Scholar] [CrossRef]
- Kuzumi, A.; Yoshizaki, A.; Matsuda, K.M.; Kotani, H.; Norimatsu, Y.; Fukayama, M.; Ebata, S.; Fukasawa, T.; Yoshizaki-Ogawa, A.; Asano, Y.; et al. Interleukin-31 promotes fibrosis and T helper 2 polarization in systemic sclerosis. Nat. Commun. 2021, 12, 5947. [Google Scholar] [CrossRef]
- Ebner, S.; Hofer, S.; Nguyen, V.A.; Furhapter, C.; Herold, M.; Fritsch, P.; Heufler, C.; Romani, N. A novel role for IL-3: Human monocytes cultured in the presence of IL-3 and IL-4 differentiate into dendritic cells that produce less IL-12 and shift Th cell responses toward a Th2 cytokine pattern. J. Immunol. 2002, 168, 6199–6207. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Stolarski, B.; Kewin, P.; Murphy, G.; Corrigan, C.J.; Ying, S.; Pitman, N.; Mirchandani, A.; Rana, B.; van Rooijen, N.; et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 2009, 183, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Yoshizaki, A.; Asano, Y.; Kadono, T.; Sato, S. Serum IL-33 levels are raised in patients with systemic sclerosis: Association with extent of skin sclerosis and severity of pulmonary fibrosis. Clin. Rheumatol. 2011, 30, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Terras, S.; Opitz, E.; Moritz, R.K.; Hoxtermann, S.; Gambichler, T.; Kreuter, A. Increased serum IL-33 levels may indicate vascular involvement in systemic sclerosis. Ann. Rheum. Dis. 2013, 72, 144–145. [Google Scholar] [CrossRef]
- Vettori, S.; Cuomo, G.; Iudici, M.; D’Abrosca, V.; Giacco, V.; Barra, G.; De Palma, R.; Valentini, G. Early systemic sclerosis: Serum profiling of factors involved in endothelial, T-cell, and fibroblast interplay is marked by elevated interleukin-33 levels. J. Clin. Immunol. 2014, 34, 663–668. [Google Scholar] [CrossRef]
- Manetti, M.; Ibba-Manneschi, L.; Liakouli, V.; Guiducci, S.; Milia, A.F.; Benelli, G.; Marrelli, A.; Conforti, M.L.; Romano, E.; Giacomelli, R.; et al. The IL1-like cytokine IL33 and its receptor ST2 are abnormally expressed in the affected skin and visceral organs of patients with systemic sclerosis. Ann. Rheum. Dis. 2010, 69, 598–605. [Google Scholar] [CrossRef]
- Rankin, A.L.; Mumm, J.B.; Murphy, E.; Turner, S.; Yu, N.; McClanahan, T.K.; Bourne, P.A.; Pierce, R.H.; Kastelein, R.; Pflanz, S. IL-33 induces IL-13-dependent cutaneous fibrosis. J. Immunol. 2010, 184, 1526–1535. [Google Scholar] [CrossRef]
- Li, L.; Zhu, H.; Zuo, X. Interleukin-33 in Systemic Sclerosis: Expression and Pathogenesis. Front. Immunol. 2018, 9, 2663. [Google Scholar] [CrossRef]
- He, R.; Yin, H.; Yuan, B.; Liu, T.; Luo, L.; Huang, P.; Dai, L.; Zeng, K. IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice. Mol. Immunol. 2017, 90, 42–49. [Google Scholar] [CrossRef]
- Casciola-Rosen, L.; Andrade, F.; Ulanet, D.; Wong, W.B.; Rosen, A. Cleavage by granzyme B is strongly predictive of autoantigen status: Implications for initiation of autoimmunity. J. Exp. Med. 1999, 190, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Forster, R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Perucha, E.; Graca, L. T follicular cells: The regulators of germinal center homeostasis. Immunol. Lett. 2022, 244, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ricard, L.; Jachiet, V.; Malard, F.; Ye, Y.; Stocker, N.; Riviere, S.; Senet, P.; Monfort, J.B.; Fain, O.; Mohty, M.; et al. Circulating follicular helper T cells are increased in systemic sclerosis and promote plasmablast differentiation through the IL-21 pathway which can be inhibited by ruxolitinib. Ann. Rheum. Dis. 2019, 78, 539–550. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Miyazaki, Y.; Yoshikawa, M.; Yoshinari, H.; Satoh, Y.; Todoroki, Y.; Nakano, K.; Satoh, M.; Smith, V.; et al. Distinctive association of peripheral immune cell phenotypes with capillaroscopic microvascular patterns in systemic sclerosis. Rheumatology 2019, 58, 2273–2283. [Google Scholar] [CrossRef]
- Ly, N.T.M.; Ueda-Hayakawa, I.; Nguyen, C.T.H.; Huynh, T.N.M.; Kishimoto, I.; Fujimoto, M.; Okamoto, H. Imbalance toward TFH 1 cells playing a role in aberrant B cell differentiation in systemic sclerosis. Rheumatology 2021, 60, 1553–1562. [Google Scholar] [CrossRef]
- Taylor, D.K.; Mittereder, N.; Kuta, E.; Delaney, T.; Burwell, T.; Dacosta, K.; Zhao, W.; Cheng, L.I.; Brown, C.; Boutrin, A.; et al. T follicular helper-like cells contribute to skin fibrosis. Sci. Transl. Med. 2018, 10, eaaf5307. [Google Scholar] [CrossRef]
- Gaydosik, A.M.; Tabib, T.; Domsic, R.; Khanna, D.; Lafyatis, R.; Fuschiotti, P. Single-cell transcriptome analysis identifies skin-specific T-cell responses in systemic sclerosis. Ann. Rheum. Dis. 2021, 80, 1453–1460. [Google Scholar] [CrossRef]
- Chizzolini, C.; Dufour, A.M.; Brembilla, N.C. Is there a role for IL-17 in the pathogenesis of systemic sclerosis? Immunol. Lett. 2018, 195, 61–67. [Google Scholar] [CrossRef]
- Lei, L.; Zhao, C.; Qin, F.; He, Z.Y.; Wang, X.; Zhong, X.N. Th17 cells and IL-17 promote the skin and lung inflammation and fibrosis process in a bleomycin-induced murine model of systemic sclerosis. Clin. Exp. Rheumatol. 2016, 34 (Suppl. S100), 14–22. [Google Scholar]
- Nakayama, W.; Jinnin, M.; Tomizawa, Y.; Nakamura, K.; Kudo, H.; Inoue, K.; Makino, K.; Honda, N.; Kajihara, I.; Fukushima, S.; et al. Dysregulated interleukin-23 signalling contributes to the increased collagen production in scleroderma fibroblasts via balancing microRNA expression. Rheumatology 2017, 56, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Wurster, A.L.; Rodgers, V.L.; Satoskar, A.R.; Whitters, M.J.; Young, D.A.; Collins, M.; Grusby, M.J. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J. Exp. Med. 2002, 196, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robak, E.; Gerlicz-Kowalczuk, Z.; Dziankowska-Bartkowiak, B.; Wozniacka, A.; Bogaczewicz, J. Serum concentrations of IL-17A, IL-17B, IL-17E and IL-17F in patients with systemic sclerosis. Arch. Med. Sci. AMS 2019, 15, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Fukayama, M.; Yoshizaki, A.; Fukasawa, T.; Ebata, S.; Kuzumi, A.; Yoshizaki-Ogawa, A.; Asano, Y.; Oba, K.; Sato, S. Interleukin (IL)-17F and IL-17E are related to fibrosis and vasculopathy in systemic sclerosis. J. Dermatol. 2020, 47, 1287–1292. [Google Scholar] [CrossRef]
- Olewicz-Gawlik, A.; Danczak-Pazdrowska, A.; Kuznar-Kaminska, B.; Gornowicz-Porowska, J.; Katulska, K.; Trzybulska, D.; Batura-Gabryel, H.; Silny, W.; Poplawski, D.; Hrycaj, P. Interleukin-17 and interleukin-23: Importance in the pathogenesis of lung impairment in patients with systemic sclerosis. Int. J. Rheum. Dis. 2014, 17, 664–670. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, W.; Xu, K.; Han, D.; Jiang, C.; Mou, K.; Li, Y.; Meng, L.; Lu, S. The elevated expression of Th17-related cytokines and receptors is associated with skin lesion severity in early systemic sclerosis. Hum. Immunol. 2015, 76, 22–29. [Google Scholar] [CrossRef]
- Vettori, S.; Barra, G.; Russo, B.; Borgia, A.; Pasquale, G.; Pellecchia, L.; Vicedomini, L.; De Palma, R. T-Cell Proapoptotic and Antifibrotic Activity Against Autologous Skin Fibroblasts in vitro Is Associated With IL-17A Axis Upregulation in Systemic Sclerosis. Front. Immunol. 2020, 11, 220. [Google Scholar] [CrossRef]
- Gabsi, A.; Heim, X.; Dlala, A.; Gati, A.; Sakhri, H.; Abidi, A.; Amri, S.; Neili, B.; Leroyer, A.S.; Bertaud, A.; et al. TH17 cells expressing CD146 are significantly increased in patients with Systemic sclerosis. Sci. Rep. 2019, 9, 17721. [Google Scholar] [CrossRef]
- Kaspi, E.; Heim, X.; Granel, B.; Guillet, B.; Stalin, J.; Nollet, M.; Bertaud-Foucault, A.; Robaglia-Schlupp, A.; Roll, P.; Cau, P.; et al. Identification of CD146 as a novel molecular actor involved in systemic sclerosis. J. Allergy Clin. Immunol. 2017, 140, 1448–1451 e6. [Google Scholar] [CrossRef]
- Lv, T.; Yang, F.; Zhang, K.; Lv, M.; Zhang, Y.; Zhu, P. The risk of circulating angiogenic T cells and subsets in patients with systemic sclerosis. Int. Immunopharmacol. 2020, 81, 106282. [Google Scholar] [CrossRef]
- Slobodin, G.; Rimar, D. Regulatory T Cells in Systemic Sclerosis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2017, 52, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Auffray, C.; Avouac, J.; Allanore, Y. Regulatory T Cells in Systemic Sclerosis. Front. Immunol. 2018, 9, 2356. [Google Scholar] [CrossRef] [PubMed]
- Radstake, T.R.; van Bon, L.; Broen, J.; Wenink, M.; Santegoets, K.; Deng, Y.; Hussaini, A.; Simms, R.; Cruikshank, W.W.; Lafyatis, R. Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFbeta expression. PLoS ONE 2009, 4, e5981. [Google Scholar] [CrossRef] [PubMed]
- Kamio, K.; Azuma, A.; Matsuda, K.; Usuki, J.; Inomata, M.; Morinaga, A.; Kashiwada, T.; Nishijima, N.; Itakura, S.; Kokuho, N.; et al. Resolution of bleomycin-induced murine pulmonary fibrosis via a splenic lymphocyte subpopulation. Respir. Res. 2018, 19, 71. [Google Scholar] [CrossRef]
- MacDonald, K.G.; Dawson, N.A.J.; Huang, Q.; Dunne, J.V.; Levings, M.K.; Broady, R. Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J. Allergy Clin. Immunol. 2015, 135, 946–955 e9. [Google Scholar] [CrossRef]
- Tamosiuniene, R.; Manouvakhova, O.; Mesange, P.; Saito, T.; Qian, J.; Sanyal, M.; Lin, Y.C.; Nguyen, L.P.; Luria, A.; Tu, A.B.; et al. Dominant Role for Regulatory T Cells in Protecting Females Against Pulmonary Hypertension. Circ. Res. 2018, 122, 1689–1702. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, C.L.; Yu, D.; Liu, Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy 2021, 76, 456–470. [Google Scholar] [CrossRef]
- Jandl, C.; Liu, S.M.; Canete, P.F.; Warren, J.; Hughes, W.E.; Vogelzang, A.; Webster, K.; Craig, M.E.; Uzel, G.; Dent, A.; et al. IL-21 restricts T follicular regulatory T cell proliferation through Bcl-6 mediated inhibition of responsiveness to IL-2. Nat. Commun. 2017, 8, 14647. [Google Scholar] [CrossRef]
- Fonseca, V.R.; Agua-Doce, A.; Maceiras, A.R.; Pierson, W.; Ribeiro, F.; Romao, V.C.; Pires, A.R.; da Silva, S.L.; Fonseca, J.E.; Sousa, A.E.; et al. Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci. Immunol. 2017, 2, eaan1487. [Google Scholar] [CrossRef]
- Wei, L.; Abraham, D.; Ong, V. The Yin and Yang of IL-17 in Systemic Sclerosis. Front. Immunol. 2022, 13, 885609. [Google Scholar] [CrossRef]
- Katsiari, C.G.; Simopoulou, T.; Alexiou, I.; Sakkas, L.I. Immunotherapy of systemic sclerosis. Hum. Vaccin. Immunother. 2018, 14, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Schmalzing, M.; Almanzar, G.; Benoit, S.; Hamm, H.; Tony, H.P.; Goebeler, M.; Prelog, M. Contribution of CD8+ T cells to inflammatory cytokine production in systemic sclerosis (SSc). Autoimmunity 2016, 49, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Spierings, J.; van Rhijn-Brouwer, F.C.C.; van Laar, J.M. Hematopoietic stem-cell transplantation in systemic sclerosis: An update. Curr. Opin. Rheumatol. 2018, 30, 541–547. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Goldmuntz, E.A.; Keyes-Elstein, L.; McSweeney, P.A.; Pinckney, A.; Welch, B.; Mayes, M.D.; Nash, R.A.; Crofford, L.J.; Eggleston, B.; et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N. Engl. J. Med. 2018, 378, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Arruda, L.C.M.; Malmegrim, K.C.R.; Lima-Junior, J.R.; Clave, E.; Dias, J.B.E.; Moraes, D.A.; Douay, C.; Fournier, I.; Moins-Teisserenc, H.; Alberdi, A.J.; et al. Immune rebound associates with a favorable clinical response to autologous HSCT in systemic sclerosis patients. Blood Adv. 2018, 2, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.A.; Robins, H.; Malhotra, S.; Howell, M.; Phippard, D.; Desmarais, C.; de Paula Alves Sousa, A.; Griffith, L.M.; Lim, N.; Nash, R.A.; et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J. Clin. Investig. 2014, 124, 1168–1172. [Google Scholar] [CrossRef]
- Daoussis, D.; Liossis, S.N. Treatment of systemic sclerosis associated fibrotic manifestations: Current options and future directions. Mediterr. J. Rheumatol. 2019, 30, 33–37. [Google Scholar] [CrossRef]
- Vlaming, M.; Bilemjian, V.; Freile, J.A.; Lourens, H.J.; van Rooij, N.; Huls, G.; van Meerten, T.; de Bruyn, M.; Bremer, E. CD20 positive CD8 T cells are a unique and transcriptionally-distinct subset of T cells with distinct transmigration properties. Sci. Rep. 2021, 11, 20499. [Google Scholar] [CrossRef]
- Melissaropoulos, K.; Daoussis, D. B cells in systemic sclerosis: From pathophysiology to treatment. Clin. Rheumatol. 2021, 40, 2621–2631. [Google Scholar] [CrossRef]
- Antonopoulos, I.; Daoussis, D.; Lalioti, M.E.; Markatseli, T.E.; Drosos, A.A.; Taraviras, S.; Andonopoulos, A.P.; Liossis, S.C. B cell depletion treatment decreases CD4+IL4+ and CD4+CD40L+ T cells in patients with systemic sclerosis. Rheumatol. Int. 2019, 39, 1889–1898. [Google Scholar] [CrossRef]
- Walker, L.S.K. The link between circulating follicular helper T cells and autoimmunity. Nat. Rev. Immunol. 2022, 22, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Ponsoye, M.; Frantz, C.; Ruzehaji, N.; Nicco, C.; Elhai, M.; Ruiz, B.; Cauvet, A.; Pezet, S.; Brandely, M.L.; Batteux, F.; et al. Treatment with abatacept prevents experimental dermal fibrosis and induces regression of established inflammation-driven fibrosis. Ann. Rheum. Dis. 2016, 75, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Boleto, G.; Guignabert, C.; Pezet, S.; Cauvet, A.; Sadoine, J.; Tu, L.; Nicco, C.; Gobeaux, C.; Batteux, F.; Allanore, Y.; et al. T-cell costimulation blockade is effective in experimental digestive and lung tissue fibrosis. Arthritis Res. Ther. 2018, 20, 197. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.; Distler, J.H.; Distler, O. The Fra-2 transgenic mouse model of systemic sclerosis. Vascul. Pharmacol. 2013, 58, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Elhai, M.; Meunier, M.; Matucci-Cerinic, M.; Maurer, B.; Riemekasten, G.; Leturcq, T.; Pellerito, R.; Von Muhlen, C.A.; Vacca, A.; Airo, P.; et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: A EUSTAR observational study. Ann. Rheum. Dis. 2013, 72, 1217–1220. [Google Scholar] [CrossRef]
- Castellvi, I.; Elhai, M.; Bruni, C.; Airo, P.; Jordan, S.; Beretta, L.; Codullo, V.; Montecucco, C.M.; Bokarewa, M.; Iannonne, F.; et al. Safety and effectiveness of abatacept in systemic sclerosis: The EUSTAR experience. Semin. Arthritis Rheum. 2020, 50, 1489–1493. [Google Scholar] [CrossRef]
- Khanna, D.; Spino, C.; Johnson, S.; Chung, L.; Whitfield, M.L.; Denton, C.P.; Berrocal, V.; Franks, J.; Mehta, B.; Molitor, J.; et al. Abatacept in Early Diffuse Cutaneous Systemic Sclerosis: Results of a Phase II Investigator-Initiated, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Arthritis Rheumatol. 2020, 72, 125–136. [Google Scholar] [CrossRef]
- Scherer, H.U.; Burmester, G.R.; Riemekasten, G. Targeting activated T cells: Successful use of anti-CD25 monoclonal antibody basiliximab in a patient with systemic sclerosis. Ann. Rheum. Dis. 2006, 65, 1245–1247. [Google Scholar] [CrossRef]
- Becker, M.O.; Bruckner, C.; Scherer, H.U.; Wassermann, N.; Humrich, J.Y.; Hanitsch, L.G.; Schneider, U.; Kawald, A.; Hanke, K.; Burmester, G.R.; et al. The monoclonal anti-CD25 antibody basiliximab for the treatment of progressive systemic sclerosis: An open-label study. Ann. Rheum. Dis. 2011, 70, 1340–1341. [Google Scholar] [CrossRef]
- Allanore, Y.; Wung, P.; Soubrane, C.; Esperet, C.; Marrache, F.; Bejuit, R.; Lahmar, A.; Khanna, D.; Denton, C.P. Investigators, A randomised, double-blind, placebo-controlled, 24-week, phase II, proof-of-concept study of romilkimab (SAR156597) in early diffuse cutaneous systemic sclerosis. Ann. Rheum. Dis. 2020, 79, 1600–1607. [Google Scholar] [CrossRef]
- Sakkas, L.I. Spotlight on tocilizumab and its potential in the treatment of systemic sclerosis. Drug. Des. Devel. Ther. 2016, 10, 2723–2728. [Google Scholar] [CrossRef]
- Khanna, D.; Lescoat, A.; Roofeh, D.; Bernstein, E.J.; Kazerooni, E.A.; Roth, M.D.; Martinez, F.; Flaherty, K.R.; Denton, C.P. Systemic Sclerosis-Associated Interstitial Lung Disease: How to Incorporate Two Food and Drug Administration-Approved Therapies in Clinical Practice. Arthritis Rheumatol. 2022, 74, 13–27. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, F.A.; Piera-Velazquez, S.; Jimenez, S.A. Tyrosine kinases in the pathogenesis of tissue fibrosis in systemic sclerosis and potential therapeutic role of their inhibition. Transl. Res. 2021, 231, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Su, X.; Han, G.; Xue, R.; Chen, Y.; Chen, Y.; Wang, H.; Yang, B.; Liang, Y.; Ji, S. JAK1/2 Inhibitor Baricitinib Improves Skin Fibrosis and Digital Ulcers in Systemic Sclerosis. Front. Med. 2022, 9, 859330. [Google Scholar] [CrossRef]
- Karatas, A.; Oz, B.; Celik, C.; Akar, Z.A.; Akkoc, R.F.; Etem, E.O.; Dagli, A.F.; Koca, S.S. Tofacitinib and metformin reduce the dermal thickness and fibrosis in mouse model of systemic sclerosis. Sci. Rep. 2022, 12, 2553. [Google Scholar] [CrossRef]
- Moriana, C.; Moulinet, T.; Jaussaud, R.; Decker, P. JAK inhibitors and systemic sclerosis: A systematic review of the literature. Autoimmun. Rev. 2022, 21, 103168. [Google Scholar] [CrossRef]
- Karalilova, R.V.; Batalov, Z.A.; Sapundzhieva, T.L.; Matucci-Cerinic, M.; Batalov, A.Z. Tofacitinib in the treatment of skin and musculoskeletal involvement in patients with systemic sclerosis, evaluated by ultrasound. Rheumatol. Int. 2021, 41, 1743–1753. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Venalis, P.; Dees, C.; Busch, N.; Zwerina, J.; Schett, G.; Distler, O.; Distler, J.H. Treatment with imatinib prevents fibrosis in different preclinical models of systemic sclerosis and induces regression of established fibrosis. Arthritis Rheum. 2009, 60, 219–224. [Google Scholar] [CrossRef]
- Liakouli, V.; Ciaffi, J.; Ursini, F.; Ruscitti, P.; Meliconi, R.; Ciccia, F.; Cipriani, P.; Giacomelli, R. Efficacy and safety of imatinib mesylate in systemic sclerosis. A systematic review and meta-analysis. Expert. Rev. Clin. Immunol. 2020, 16, 931–942. [Google Scholar] [CrossRef]
- Beurier, P.; Ricard, L.; Eshagh, D.; Malard, F.; Siblany, L.; Fain, O.; Mohty, M.; Gaugler, B.; Mekinian, A. TFH cells in systemic sclerosis. J. Transl. Med. 2021, 19, 375. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Nakayamada, S.; Yamagata, K.; Ohkubo, N.; Iwata, S.; Inoue, Y.; Zhang, M.; Zhang, T.; Kanda Satoh, Y.; Shan, Y.; et al. Conversion of T Follicular Helper Cells to T Follicular Regulatory Cells by Interleukin-2 Through Transcriptional Regulation in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2021, 73, 132–142. [Google Scholar] [CrossRef]
- Von Spee-Mayer, C.; Siegert, E.; Abdirama, D.; Rose, A.; Klaus, A.; Alexander, T.; Enghard, P.; Sawitzki, B.; Hiepe, F.; Radbruch, A.; et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2016, 75, 1407–1415. [Google Scholar] [CrossRef]
- He, J.; Zhang, X.; Wei, Y.; Sun, X.; Chen, Y.; Deng, J.; Jin, Y.; Gan, Y.; Hu, X.; Jia, R.; et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 2016, 22, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xiao, Y.; Zeng, Z.; Shi, Y.; Tang, B.; Long, H.; Kanekura, T.; Wang, J.; Wu, H.; Zhao, M.; et al. All-Trans Retinoic Acid Induces CD4+CD25+FOXP3+ Regulatory T Cells by Increasing FOXP3 Demethylation in Systemic Sclerosis CD4+ T Cells. J. Immunol. Res. 2018, 2018, 8658156. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Asano, Y.; Saigusa, R.; Yamashita, T.; Taniguchi, T.; Takahashi, T.; Ichimura, Y.; Toyama, T.; Yoshizaki, A.; Sato, S.; et al. Regulation of skin fibrosis by RALDH1-producing dermal dendritic cells via retinoic acid-mediated regulatory T cell induction: A role in scleroderma. J. Dermatol. Sci. 2020, 97, 125–134. [Google Scholar] [CrossRef]
- Urzainqui, A.; Martinez del Hoyo, G.; Lamana, A.; de la Fuente, H.; Barreiro, O.; Olazabal, I.M.; Martin, P.; Wild, M.K.; Vestweber, D.; Gonzalez-Amaro, R.; et al. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J. Immunol. 2007, 179, 7457–7465. [Google Scholar] [CrossRef]
- Perez-Frias, A.; Gonzalez-Tajuelo, R.; Nunez-Andrade, N.; Tejedor, R.; Garcia-Blanco, M.J.; Vicente-Rabaneda, E.; Castaneda, S.; Gamallo, C.; Silvan, J.; Esteban-Villafruela, A.; et al. Development of an autoimmune syndrome affecting the skin and internal organs in P-selectin glycoprotein ligand 1 leukocyte receptor-deficient mice. Arthritis Rheumatol. 2014, 66, 3178–3189. [Google Scholar] [CrossRef]
- Gonzalez-Tajuelo, R.; de la Fuente-Fernandez, M.; Morales-Cano, D.; Munoz-Callejas, A.; Gonzalez-Sanchez, E.; Silvan, J.; Serrador, J.M.; Cadenas, S.; Barreira, B.; Espartero-Santos, M.; et al. Spontaneous Pulmonary Hypertension Associated With Systemic Sclerosis in P-Selectin Glycoprotein Ligand 1-Deficient Mice. Arthritis Rheumatol. 2020, 72, 477–487. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.; Lei, L.; Tao, Z.; Zheng, L.; Wen, J.; Li, X. Effects of thalidomide on Th17, Treg cells and TGF-beta1/Smad3 pathway in a mouse model of systemic sclerosis. Int. J. Rheum. Dis. 2020, 23, 406–419. [Google Scholar] [CrossRef]
- Kano, M.; Kobayashi, T.; Date, M.; Tennichi, M.; Hamaguchi, Y.; Strasser, D.S.; Takehara, K.; Matsushita, T. Attenuation of murine sclerodermatous models by the selective S1P1 receptor modulator cenerimod. Sci. Rep. 2019, 9, 658. [Google Scholar] [CrossRef] [PubMed]
- Orvain, C.; Boulch, M.; Bousso, P.; Allanore, Y.; Avouac, J. Is There a Place for Chimeric Antigen Receptor-T Cells in the Treatment of Chronic Autoimmune Rheumatic Diseases? Arthritis Rheumatol. 2021, 73, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Mukhatayev, Z.; Ostapchuk, Y.O.; Fang, D.; Le Poole, I.C. Engineered antigen-specific regulatory T cells for autoimmune skin conditions. Autoimmun. Rev. 2021, 20, 102761. [Google Scholar] [CrossRef]
- Reighard, S.D.; Cranert, S.A.; Rangel, K.M.; Ali, A.; Gyurova, I.E.; de la Cruz-Lynch, A.T.; Tuazon, J.A.; Khodoun, M.V.; Kottyan, L.C.; Smith, D.F.; et al. Therapeutic Targeting of Follicular T Cells with Chimeric Antigen Receptor-Expressing Natural Killer Cells. Cell Rep. Med. 2020, 1, 100003. [Google Scholar] [CrossRef] [PubMed]
- De Paoli, F.V.; Nielsen, B.D.; Rasmussen, F.; Deleuran, B.; Sondergaard, K. Abatacept induces clinical improvement in patients with severe systemic sclerosis. Scand. J. Rheumatol. 2014, 43, 342–345. [Google Scholar] [CrossRef] [PubMed]

| Agent | Action, Cells | Clinical Effects | Reference |
|---|---|---|---|
| ASCT | Renewal and reset of immune system. | Improves skin fibrosis and vasculopathy and stabilizes lung function. | [93,94,95] |
| Mycophenolate mofetil | Non-specific immunosuppressant of T cells and B cells. | Improves skin fibrosis and stabilizes lung function. | [91,97] |
| Cyclophosphamide | Non-specific immunosuppressant. | Stabilizes lung function. | [91] |
| Rituximab | anti-CD20 moAb. Eliminates B cells, and a subset of T cells. | Improves skin fibrosis and stabilizes lung function. | [98,99,100] |
| Abatacept | CTLA4-Ig construct. Inhibits activated Tcells, and antigen-presenting cells. Decreases TFH cells and CD4+CTLs. | Improves skin score. | [21,105,106,107,135] |
| Basiliximab | Anti-CD25 moAb. Inhibits activated T cells. | Improves skin. | [108,109] |
| Romilkimab | Bispecific moAb against IL-4/IL-13 which are derived from TH2 cells, mast cells eosinophils, innate lymphoid cells. Inhibits M2 macrophages, and myofibroblasts. | Decreases skin score. | [110] |
| Anti-IL-21 moAb | Inhibits IL-21 which is produced by TH17 cells and TFH cells. Inhibits TH17 cells. Suppresses TFH cells. | Not studied in SSc. | [67] |
| Tocilizumab | moAb against IL-6R. Inhibits B cell differentiation, M2 macrophages, TH17 polarization, and myofibroblast activation. | Decreases skin score, stabilizes lung function. | [111,112] |
| Tofacitinib | Pan-JAK inhibitor. Inhibits activation of T cells, macrophages, and fibroblasts | Decreases skin score and promotes healing of DUs | [117,118] |
| Baricitinib | JAK1/2 inhibitor. Inhibits activation of T cells, macrophages, and fibroblasts. | Decreases skin score and promotes healing of DUs. | [115,117] |
| Ruxolitinib | JAK1/2 inhibitor. Inhibits activation of T cells, macrophages, and fibroblasts. Inhibits TFH cells. | Not studied in SSc. | [64] |
| Itacitinib | JAK2 inhibitor. Inhibits activation of T cells, macrophages, and fibroblasts. | Is being studied in SSc. | [115] |
| Low-dose IL-2 | Increases Tregs. Decreases TFH cells. | Not studied in SSc. | [123,124] |
| Thalidomide | Increases Tregs. Inhibits TH17 cells. | Not studied in SSc. | [130] |
| CAR-T cells | Against various selected cell targets. | Not studied in SSc. | [132,133,134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakkas, L.I.; Bogdanos, D.P. The Role of T Cells in Systemic Sclerosis: An Update. Immuno 2022, 2, 534-547. https://doi.org/10.3390/immuno2030034
Sakkas LI, Bogdanos DP. The Role of T Cells in Systemic Sclerosis: An Update. Immuno. 2022; 2(3):534-547. https://doi.org/10.3390/immuno2030034
Chicago/Turabian StyleSakkas, Lazaros I., and Dimitrios P. Bogdanos. 2022. "The Role of T Cells in Systemic Sclerosis: An Update" Immuno 2, no. 3: 534-547. https://doi.org/10.3390/immuno2030034
APA StyleSakkas, L. I., & Bogdanos, D. P. (2022). The Role of T Cells in Systemic Sclerosis: An Update. Immuno, 2(3), 534-547. https://doi.org/10.3390/immuno2030034