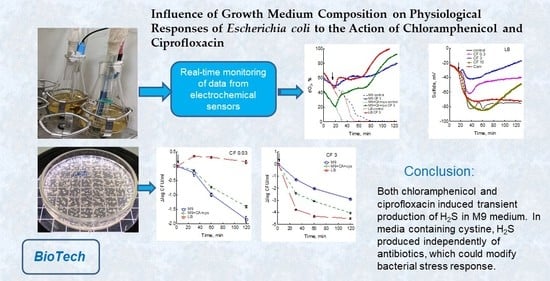
Influence of Growth Medium Composition on Physiological Responses of Escherichia coli to the Action of Chloramphenicol and Ciprofloxacin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Real-Time Monitoring of Dissolved Oxygen (dO2), pH and Extracellular K+ and Sulfide
2.3. Determination of ATP, NAD+/NADH Ratio and Membrane Potential
2.4. Determination of H2S in the Gas Phase
2.5. Study of Cell Viability and β-Galactosidase Activity
2.6. Statistical Analysis of the Data
3. Results
3.1. Effect of Chloramphenicol and Ciprofloxacin on the Growth and Respiration of E. coli during Cultivation in Different Media
3.2. Medium Composition Affects Ciprofloxacin-Induced Changes in E. coli Energetics
3.3. Sulfide Production under the Action of Antibiotics in Different Media
3.4. Influence of Medium Composition on SOS Response and Viability of E. coli upon Exposure to Ciprofloxacin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baquero, F.; Levin, B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat. Rev. Microbiol. 2021, 19, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Keren, I.; Wu, Y.; Inocencio, J.; Mulcahy, L.R.; Lewis, K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 2013, 339, 1213–1216. [Google Scholar] [CrossRef]
- Liu, Y.; Imlay, J.A. Cell death from antibiotics without the involvement of reactive oxygen species. Science 2013, 339, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Diagnosing oxidative stress in bacteria: Not as easy as you might think. Curr. Opin. Microbiol. 2015, 24, 124–131. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.Y.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef] [PubMed]
- Lobritz, M.A.; Belenky, P.; Porter, C.B.M.; Gutierrez, A.; Yang, J.H.; Schwarz, E.G.; Dwyer, D.J.; Khalil, A.S.; Collins, J.J. Antibiotic efficacy is linked to bacterial cellular respiration. Proc. Natl. Acad. Sci. USA 2015, 112, 8173–8180. [Google Scholar] [CrossRef]
- Yang, J.H.; Bening, S.C.; Collins, J.J. Antibiotic efficacy–context matters. Curr. Opin. Microbiol. 2017, 39, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Mironov, A.; Seregina, T.; Nagornykh, M.; Luhachack, L.G.; Korolkova, N.; Lopes, L.E.; Kotova, V.; Zavilgelsky, G.; Shakulov, R.; Shatalin, K.; et al. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli. Proc. Natl. Acad. Sci. USA 2017, 114, 6022–6027. [Google Scholar] [CrossRef]
- Shatalin, K.; Nuthanakanti, A.; Kaushik, A.; Shishov, D.; Peselis, A.; Shamovsky, I.; Pani, B.; Lechpammer, M.; Vasilyev, N.; Shatalina, E.; et al. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 2021, 372, 1169–1175. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, lethality and their contributions to antibiotic resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Hong, Y.; Li, Q.; Gao, Q.; Xie, J.; Huang, H.; Drlica, K.; Zhao, X. Reactive oxygen species play a dominant role in all pathways of rapid quinolone-mediated killing. J. Antimicrob. Chemother. 2020, 75, 576–585. [Google Scholar] [CrossRef]
- Smirnova, G.; Muzyka, N.; Lepekhina, E.; Oktyabrsky, O. Roles of the glutathione- and thioredoxin-dependent systems in the Escherichia coli responses to ciprofloxacin and ampicillin. Arch. Microbiol. 2016, 198, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, G.V.; Tyulenev, A.V.; Muzyka, N.G.; Peters, M.A.; Oktyabrsky, O.N. Ciprofloxacin provokes SOS-dependent changes in respiration and membrane potential and causes alterations in the redox status of Escherichia coli. Res. Microbiol. 2017, 168, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, G.V.; Tyulenev, A.V.; Muzyka, N.G.; Oktyabrsky, O.N. Study of the contribution of active defense mechanisms to ciprofloxacin tolerance in Escherichia coli growing at different rates. Antonie Van Leeuwenhoek 2022, 115, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, G.V.; Oktyabrsky, O.N. Relationship between Escherichia coli growth rate and bacterial susceptibility to ciprofloxacin. FEMS Microbiol. Lett. 2018, 365, fnx254. [Google Scholar] [CrossRef]
- Tyulenev, A.; Smirnova, G.; Muzyka, N.; Ushakov, V.; Oktyabrsky, O. The role of sulfides in stress-induced changes of Eh in Escherichia coli cultures. Bioelectrochemistry 2018, 121, 11–17. [Google Scholar] [CrossRef]
- Smirnova, G.V.; Tyulenev, A.V.; Bezmaternykh, K.V.; Muzyka, N.G.; Ushakov, V.Y.; Oktyabrsky, O.N. Cysteine homeostasis under inhibition of protein synthesis in Escherichia coli cells. Amino Acids 2019, 51, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Tyulenev, A.V.; Smirnova, G.V.; Muzyka, N.G.; Oktyabrsky, O.N. Study of the early response of Escherichia coli lpcA and ompF mutants to ciprofloxacin. Res. Microbiol. 2022, 173, 1033954. [Google Scholar] [CrossRef]
- Park, S.; Imlay, J.A. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 2003, 185, 1942–1950. [Google Scholar] [CrossRef]
- Imlay, K.R.C.; Korshunov, S.; Imlay, J.A. The physiological roles and adverse effects of the two cystine importers of Escherichia coli. J. Bacteriol. 2015, 197, 3629–3644. [Google Scholar] [CrossRef] [PubMed]
- Volkert, M.R.; Gately, F.H.; Hajec, L.I. Expression of DNA damage-inducible genes of Escherichia coli upon treatment with methylating, ethylating and propylating agents. Mut. Res. 1989, 217, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1972. [Google Scholar]
- Leonardo, M.R.; Dailly, Y.; Clark, D.P. Role of NAD in regulating the adhE gene in Escherichia coli. J. Bacteriol. 1996, 178, 6013–6018. [Google Scholar] [CrossRef] [PubMed]
- Wickens, H.J.; Pinney, R.J.; Mason, D.J.; Gant, V.A. Flow cytometric investigation of filamentation, membrane patency and membrane potential in Escherichia coli following ciprofloxacin exposure. Antimicrob. Agents Chemother. 2000, 44, 682–687. [Google Scholar] [CrossRef]
- Maslowska, K.H.; Makiela-Dzbenska, K.; Fijalkowska, I.J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019, 60, 368–384. [Google Scholar] [CrossRef]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef]
- Theodore, A.; Lewis, K.; Vulic, M. Tolerance of Escherichia coli to fluoroquinolone antibiotics depends on specific components of the SOS response pathway. Genetics 2013, 195, 1265–1276. [Google Scholar] [CrossRef]
- Lewin, C.S.; Morrissey, I.; Smith, J.T. The mode of action of quinolones: The paradox in activity of low and high concentrations and activity in the anaerobic environment. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 240–248. [Google Scholar] [CrossRef]
- Pontes, M.H.; Groisman, E.A. Physiological basis for nonheritable antibiotic resistance. mBio 2020, 11, e00817-20. [Google Scholar] [CrossRef]
- Luan, G.; Hong, Y.; Drlica, K.; Zhao, X. Suppression of reactive oxygen species accumulation accounts for paradoxical bacterial survival at high quinolone concentration. Antimicrob. Agents Chemother. 2018, 62, e01622-17. [Google Scholar] [CrossRef] [PubMed]
- Kredich, N.M. The molecular basis for positive regulation of cys promoters in Salmonella typhimurium and Escherichia coli. Mol. Microbiol. 1992, 6, 2747–2753. [Google Scholar] [CrossRef]
- Korshunov, S.; Imlay, K.R.C.; Imlay, J.A. Cystine import is a valuable but risky process whose hazards Escherichia coli minimizes by inducing a cysteine exporter. Mol. Microbiol. 2020, 113, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Weisemann, J.M.; Weinstock, G.M. Mutations at the cysteine codons of the recA gene of Escherichia coli. DNA 1988, 7, 389–398. [Google Scholar] [CrossRef]
- Korshunov, S.; Imlay, K.R.C.; Imlay, J.A. The cytochrome bd oxidase of Escherichia coli prevents respiratory inhibition by endogenous and exogenous hydrogen sulfide. Mol. Microbiol. 2016, 101, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Borisov, V.B.; Falabella, M.; Colaço, H.G.; Tinajero-Trejo, M.; Poole, R.K.; Vicente, J.B.; Sarti, P.; Giuffrè, A. The terminal oxidase cytochrome bd promotes sulfide-resistant bacterial respiration and growth. Sci. Rep. 2016, 6, 23788. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnova, G.; Tyulenev, A.; Muzyka, N.; Ushakov, V.; Samoilova, Z.; Oktyabrsky, O. Influence of Growth Medium Composition on Physiological Responses of Escherichia coli to the Action of Chloramphenicol and Ciprofloxacin. BioTech 2023, 12, 43. https://doi.org/10.3390/biotech12020043
Smirnova G, Tyulenev A, Muzyka N, Ushakov V, Samoilova Z, Oktyabrsky O. Influence of Growth Medium Composition on Physiological Responses of Escherichia coli to the Action of Chloramphenicol and Ciprofloxacin. BioTech. 2023; 12(2):43. https://doi.org/10.3390/biotech12020043
Chicago/Turabian StyleSmirnova, Galina, Aleksey Tyulenev, Nadezda Muzyka, Vadim Ushakov, Zoya Samoilova, and Oleg Oktyabrsky. 2023. "Influence of Growth Medium Composition on Physiological Responses of Escherichia coli to the Action of Chloramphenicol and Ciprofloxacin" BioTech 12, no. 2: 43. https://doi.org/10.3390/biotech12020043
APA StyleSmirnova, G., Tyulenev, A., Muzyka, N., Ushakov, V., Samoilova, Z., & Oktyabrsky, O. (2023). Influence of Growth Medium Composition on Physiological Responses of Escherichia coli to the Action of Chloramphenicol and Ciprofloxacin. BioTech, 12(2), 43. https://doi.org/10.3390/biotech12020043