Improved Antigen Detection of Male-Only Dirofilaria immitis Infections in Canine Serum after Heat Treatment for Immune Complex Dissociation
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Sampling
4.2. Study Design
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCall, J.W.; McTier, T.L.; Supakorndej, N.; Ricketts, R.P.; Dzimianski, M.T. Further characterization of the sensitivity of several commercially available heartworm antigen test kits. In Proceedings of the North American Veterinary Conference, Orlando, FL, USA, 15–20 January 1994; Volume 8, pp. 461–463. [Google Scholar]
- Courtney, C.H. Comparison of tests for immunodiagnosis of canine dirofilariasis. In Proceedings of the Heartworm Symposium’86, New Orleans, LA, USA, 21–23 March 1986; Otto, G.F., Ed.; American Heartworm Society: Washington, DC, USA, 1986; pp. 77–82. [Google Scholar]
- Ely, M.L.; Courtney, C.H. Sensitivity and specificity of Filarochek heartworm antigen test and Dirotect heartworm antibody test for immunodiagnosis of canine dirofilariasis. J. Am. Anim. Hosp. Assoc. 1987, 23, 367–371. [Google Scholar]
- Courtney, C.H.; Zeng, Q.Y.; Bean, E.S. Sensitivity and specificity of the DiroChek heartworm antigen test for immunodiagnosis of canine dirofilariasis and a comparison with other immunodiagnostic tests. J. Am. Anim. Hosp. Assoc. 1988, 24, 27–32. [Google Scholar]
- Courtney, C.H.; Zeng, Q.Y. The structure of heartworm populations in dogs and cats in Florida. In Proceedings of the Heartworm Symposium ’89, Charleston, SC, USA, 17–19 March 1989; Otto, G.F., Ed.; American Heartworm Society: Washington, DC, USA, 1989; pp. 1–6. [Google Scholar]
- Courtney, C.H.; Zeng, Q.Y.; Tonelli, Q. Sensitivity and specificity of the CITE heartworm antigen test and a comparison with the DiroChek heartworm antigen test. J. Am. Anim. Hosp. Assoc. 1990, 26, 623–628. [Google Scholar]
- Courtney, C.H.; Cornell, J.A. Evaluation of heartworm immunodiagnostic tests. J. Am. Vet. Med. Assoc. 1990, 197, 724–729. [Google Scholar] [PubMed]
- Tseggai, T.; Zeng, Q.Y.; Cochran, D.; Courtney, C.; Mackowiak, M. Comparisons of currently used canine heartworm antigen detection kits with VetRed. In Proceedings of the 39th Annual American Association of Veterinary Parasitologists Meeting (AAVP), San Francisco, CA, USA, 9–12 July 1994; Volume 91, p. 62. [Google Scholar]
- Martini, M.; Capelli, G.; Poglayen, G.; Bertotti, F.; Turilli, C. The validity of some haematological and ELISA methods for the diagnosis of canine heartworm disease. Vet. Res. Commun. 1996, 20, 331–339. [Google Scholar] [CrossRef]
- Weil, G.J. Dirofilaria immitis: Identification and partial characterization of parasite antigens in the serum of infected dogs. Exp. Parasitol. 1987, 64, 244–251. [Google Scholar] [CrossRef]
- McCall, J.W.; Supakorndej, N.; McTier, T.L.; Dzimianski, M.T.; Ricketts, R.P. Commercial heartworm test kits detect infections with a single adult female worm but not those with numerous adult male worms only. In Proceedings of the American Association of Veterinary Parasitologists, Minneapolis, MN, USA, 17–20 July 1993. #44. [Google Scholar]
- McCall, J.W.; Supakorndej, N.; Donoghue, A.R.; Turnbull, R.K.; Radecki, S.V. Evaluation of the performance of canine heartworm antigen test kits licensed for use by veterinarians and canine heartworm antigen tests conducted by diagnostic laboratories, and canine heartworm antigen tests conducted by diagnostic laboratories. In Proceedings of the Recent Advances in Heartworm Disease Symposium’01, San Antonio, TX, USA, 20–22 April 2001; Seward, L.E., Knight, D.H., Eds.; American Heartworm Society: Batavia, IL, USA, 2001; pp. 135–140. [Google Scholar]
- McTier, T.L.; McCall, J.W.; Supakorndej, N. Comparative Evaluation of the sensitivity of adult heartworm antigen test kits using whole blood and/or Serum. In Proceedings of the 39th Annual American Association of Veterinary Parasitologists Meeting (AAVP), San Francisco, CA, USA, 9–12 July 1994; Volume 92, p. 62. [Google Scholar]
- Chandrashekar, R.; Beall, M.J.; Saucier, J.; O’Connor, T.; McCall, J.W.; McCall, S.D. Experimental Dirofilaria immitis infection in dogs: Effects of doxycycline and advantage multi® administration on immature adult parasites. Vet. Parasitol. 2014, 206, 93–98. [Google Scholar] [CrossRef]
- McCall, J.W. Heartworm strains and testing preventive drugs. Am. Heartworm Soc. Bull. 2011, 38, 12–13. [Google Scholar]
- Vidyashankar, A.N.; Castro, P.D.; Kaplan, R.M. A statistical approach for evaluating the effectiveness of heartworm preventive drugs: What does 100% efficacy really mean? Parasites Vectors 2017, 10, 97–108. [Google Scholar] [CrossRef] [Green Version]
- McTier, T.L.; Holzmer, S.; Kryda, K.; Mahabir, S.; McCall, J.W.; Trombley, J.; Maeder, S.J. Comparative preventive efficacy of ProHeart® 12, Heartgard® Plus and Interceptor® Plus against a macrocyclic lactone-resistant strain (JYD-34) of heartworm (Dirofilaria immitis) in dogs. Parasit. Vectors 2021, 14, 226. [Google Scholar] [CrossRef]
- Nelson, C.T.; McCall, J.W.; Jones, S.; Moorhead, A. (Eds.) Current Canine Guidelines for the Prevention, Diagnosis and Management of Heartworm (Dirofilaria immitis) Infection in Dogs. 2020. Available online: https://www.heartwormsociety.org/veterinary-resources/american-heartworm-society-guidlines (accessed on 26 April 2021).
- Otto, G.F. The significance of microfilaremia in the diagnosis of heartworm infection. In Proceedings of the Heartworm Symposium ’77, Atlanta, GA, USA, 18–20 March 1977; Morgan, H.C., Otto, G.F., Eds.; Veterinary Medicine Publishing Co.: Bonner Springs, KS, USA, 1978; pp. 77–82. [Google Scholar]
- Grieve, R. Advances in the immunologic diagnosis of Dirofilaria immitis infection. Sem. Vet. Med. Surg. 1987, 2, 4–14. [Google Scholar]
- Rawlings, C.A.; Dawe, D.L.; McCall, J.W.; Keith, J.C.; Prestwood, A.K. Four types of occult Dirofilaria immitis infection in dogs. J. Am. Vet. Med. Assoc. 1982, 180, 1323–1326. [Google Scholar]
- Gruntmeir, J.M.; Thompson, N.M.; Long, M.T.; Blagburn, B.L.; Walden, H.D. Detection of heartworm antigen without cross-reactivity to helminths and protozoa following heat treatment of canine serum. Parasit. Vectors 2021, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Rishniw, M.; Schukken, Y.; Greiner, E. Sex ratios of Dirofilaria immitis in naturally infected dogs show female bias at low worm intensities. Res. Vet. Sci. 2012, 93, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Gruntmeir, J.M.; Long, M.T.; Blagburn, B.L.; Walden, H.S. Canine heartworm and heat treatment: An evaluation using a well based enzyme-linked immunosorbent assay (ELISA) and canine sera with confirmed heartworm infection status. Vet. Parasitol. 2020, 283, 109169. [Google Scholar] [CrossRef] [PubMed]
- Little, S.E.; Munzing, C.; Heise, S.R.; Allen, K.E.; Starkey, L.A.; Johnson, E.M.; Meinkoth, J.; Reichard, M.V. Pre-treatment with heat facilitates detection of antigen of Dirofilaria immitis in canine samples. Vet. Parasitol. 2014, 203, 250–252. [Google Scholar] [CrossRef]
- Little, S.E.; Raymond, M.R.; Thomas, J.E.; Gruntmeir, J.; Hostetler, J.A.; Meinkoth, J.H.; Blagburn, B.L. Heat treatment prior to testing allows detection of antigen of Dirofilaria immitis in feline serum. Parasit. Vectors 2014, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Little, S.; Saleh, M.; Wohltjen, M.; Nagamori, Y. Prime detection of Dirofilaria immitis: Understanding the influence of blocked antigen on heartworm test performance. Parasites Vectors 2018, 11, 186. [Google Scholar] [CrossRef] [Green Version]
- Gruntmeir, J.M.; Abbott, J.R.; Kima, P.E.; Long, M.T.; Blagburn, B.L.; Walden, H.D. Increasing temperature denatures canine IgG reducing its ability to inhibit heartworm antigen detection. Parasit. Vectors, 2023; in press. [Google Scholar]
- Drake, J.; Gruntmeir, J.; Merritt, H.; Allen, L.; Little, S.E. False negative antigen tests in dogs infected with heartworm and placed on macrocyclic lactone preventives. Parasites Vectors 2015, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Ames, M.K.; VanVranken, P.; Evans, C.; Atkins, C.E. Non-Arsenical heartworm adulticidal therapy using topical moxidectin-imidacloprid and doxycycline: A prospective case series. Vet. Parasitol. 2020, 282, 109099. [Google Scholar] [CrossRef]
- Paterson, T.; Fernandez, C.; Burnett, P.J.; Lessey, L.; Hockley, T.; Hagen, R.; Coomansingh, C.; Sharma, B.; Chandrashekar, R.; Schaper, R. Heartworm control in Grenada, West Indies: Results of a field study using imidacloprid 10%+ moxidectin 2.5% and doxycycline for naturally-acquired Dirofilaria immitis infections. Vet. Parasitol. 2020, 284, 109194. [Google Scholar] [CrossRef] [PubMed]
- Szatmári, V.; van Leeuwen, M.W.; Piek, C.J.; Venco, L. False positive antigen test for Dirofilaria immitis after heat treatment of the blood sample in a microfilaremic dog infected with Acanthocheilonema dracunculoides. Parasit. Vectors 2020, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Gruntmeir, J.M.; Adolph, C.B.; Thomas, J.E.; Reichard, M.V.; Blagburn, B.L.; Little, S.E. Increased detection of Dirofilaria immitis antigen in cats after heat pretreatment of samples. J. Fel. Med. Surg. 2017, 19, 1013–1016. [Google Scholar] [CrossRef]
- McTier, T.L.; McCall, J.W.; Supakorndej, N. Features of adult heartworm antigen test kits. In Proceedings of the Heartworm Symposium ‘95, Auburn, AL, USA, 31 March–2 April 1995; Soll, M.D., Knight, D.S., Eds.; American Heartworm Society: Batavia, IL, USA, 1995; pp. 115–120. [Google Scholar]
- Kotani, T.; Powers, K.G. Developmental stages of Dirofilaria immitis in the dog. Am. J. Vet. Res. 1982, 43, 2199–2206. [Google Scholar]
- Kume, S.; Itagaki, S. On the life-cycle of Dirofilaria immtis in the dog as the final host. Br. Vet. J. 1955, 111, 16–24. [Google Scholar] [CrossRef]
- Orihel, T.C. Morphology of the larval stages of Dirofilaria immitis in the dog. J. Parasitol. 1961, 147, 251–262. [Google Scholar] [CrossRef]
- Henry, L.; Brunson, K.; Walden, H.; Wenzlow, N.; Beachboard, S.; Barr, K.; Long, M. Comparison of six commercial antigen kits for detection of Dirofilaria immitis infections in canines with necropsy-confirmed heartworm status. Vet. Parasitol. 2018, 254, 178–182. [Google Scholar] [CrossRef] [PubMed]
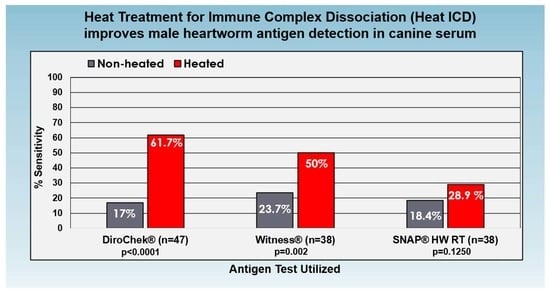
| DCK HW | WIT HW | SNP HW | ||||
|---|---|---|---|---|---|---|
| Non-Heat ICD | Heat ICD | Non-Heat ICD | Heat ICD | Non-Heat ICD | Heat ICD | |
| Mature Males #Positive/#Tested | 8/47 | 29/47 | ||||
| % Sensitivity | 17.0% | 61.7% | ||||
| Change in Sensitivity, p = value | +44.7%, p < 0.0001 | |||||
| Mature Males #Positive/#Tested | 8/38 | 24/38 | 9/38 | 19/38 | 7/38 | 11/38 |
| % Sensitivity | 21.1% | 63.2% | 23.7% | 50.0% | 18.4% | 28.9% |
| Change in Sensitivity, p = value | +42.1%, p < 0.0001 | +26.3%, p = 0.002 | +10.5%, p = 0.1250 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruntmeir, J.; Long, M.; Blagburn, B.; Walden, H. Improved Antigen Detection of Male-Only Dirofilaria immitis Infections in Canine Serum after Heat Treatment for Immune Complex Dissociation. Parasitologia 2023, 3, 79-86. https://doi.org/10.3390/parasitologia3010010
Gruntmeir J, Long M, Blagburn B, Walden H. Improved Antigen Detection of Male-Only Dirofilaria immitis Infections in Canine Serum after Heat Treatment for Immune Complex Dissociation. Parasitologia. 2023; 3(1):79-86. https://doi.org/10.3390/parasitologia3010010
Chicago/Turabian StyleGruntmeir, Jeff, Maureen Long, Byron Blagburn, and Heather Walden. 2023. "Improved Antigen Detection of Male-Only Dirofilaria immitis Infections in Canine Serum after Heat Treatment for Immune Complex Dissociation" Parasitologia 3, no. 1: 79-86. https://doi.org/10.3390/parasitologia3010010
APA StyleGruntmeir, J., Long, M., Blagburn, B., & Walden, H. (2023). Improved Antigen Detection of Male-Only Dirofilaria immitis Infections in Canine Serum after Heat Treatment for Immune Complex Dissociation. Parasitologia, 3(1), 79-86. https://doi.org/10.3390/parasitologia3010010