Ultrasonication-Assisted Aqueous Extraction of Waste Orange Peel Polyphenols: Optimization of Process Variables and Effect on Extract Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals–Reagents
2.2. Waste Orange Peel (WOP) Collection
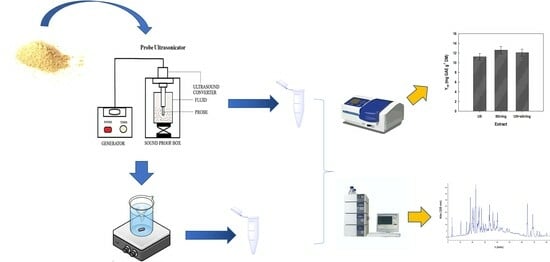
2.3. Ultrasonication-Assisted Aqueous Extraction
2.4. Batch Stirred-Tank Aqueous Extraction
2.5. Experimental Design and Response Surface Methodology
2.6. Total Polyphenol Yield (YTP), Ferric-Reducing Power (PR), and Antiradical Activity (AAR) Determination
2.7. Chromatographic Determinations
2.8. Statistics–Data Processing
3. Results and Discussion
3.1. Single-Factor Examination
3.2. Design of Experiment and Response Surface Optimization
3.3. Comparative Evaluation
3.4. Profile of Polyphenolic Metabolites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Monteiro, A.R.; Battisti, A.P.; Valencia, G.A.; de Andrade, C.J. The production of high-added-value bioproducts from non-conventional biomasses: An overview. Biomass 2023, 3, 123–137. [Google Scholar] [CrossRef]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals derived from agricultural residues and their valuable properties and applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef] [PubMed]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Sathiya Seelan, J.S. Value-added metabolites from agricultural waste and application of green extraction techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- Ademosun, A.O. Citrus peels odyssey: From the waste bin to the lab bench to the dining table. Appl. Food Res. 2022, 2, 100083. [Google Scholar] [CrossRef]
- Mohsin, A.; Hussain, M.H.; Zaman, W.Q.; Mohsin, M.Z.; Zhang, J.; Liu, Z.; Tian, X.; Salim-ur-Rehman; Khan, I.M.; Niazi, S. Advances in sustainable approaches utilizing orange peel waste to produce highly value-added bioproducts. Crit. Rev. Biotech. 2022, 42, 1284–1303. [Google Scholar] [CrossRef] [PubMed]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Phytochemical characteristics of citrus peel and effect of conventional and nonconventional processing on phenolic compounds: A review. Food Rev. Inter. 2017, 33, 587–619. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, C.; Ma, Y.; Jia, M. Degradation behavior of polyphenols in model aqueous extraction system based on mechanical and sonochemical effects induced by ultrasound. Separ. Purif. Technol. 2020, 247, 116967. [Google Scholar] [CrossRef]
- Kimura, T.; Sakamoto, T.; Leveque, J.-M.; Sohmiya, H.; Fujita, M.; Ikeda, S.; Ando, T. Standardization of ultrasonic power for sonochemical reaction. Ultrason. Sonochem. 1996, 3, S157–S161. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Athanasiadis, V.; Palaiogiannis, D.; Lalas, S.I.; Makris, D.P. Valorization of waste orange peels: Aqueous antioxidant polyphenol extraction as affected by organic acid addition. Beverages 2022, 8, 71. [Google Scholar] [CrossRef]
- Mota, I.; Rodrigues Pinto, P.C.; Novo, C.; Sousa, G.; Guerreiro, O.; Guerra, A.R.; Duarte, M.F.; Rodrigues, A.E. Extraction of polyphenolic compounds from Eucalyptus globulus bark: Process optimization and screening for biological activity. Ind. Eng. Chem. Res. 2012, 51, 6991–7000. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulou, M.A.; Kefalas, P.; Kokkalou, E.; Assimopoulou, A.N.; Papageorgiou, V.P. Analysis of antioxidant compounds in sweet orange peel by HPLC–diode array detection–electrospray ionization mass spectrometry. Biomed. Chrom. 2005, 19, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, E.; Rosselló, C.; Eim, V.; Ratti, C.; Simal, S. Ultrasound-assisted aqueous extraction of biocompounds from orange byproduct: Experimental kinetics and modeling. Antioxidants 2020, 9, 352. [Google Scholar] [CrossRef]
- Dujmić, F.; Kovačević Ganić, K.; Ćurić, D.; Karlović, S.; Bosiljkov, T.; Ježek, D.; Vidrih, R.; Hribar, J.; Zlatić, E.; Prusina, T. Non-thermal ultrasonic extraction of polyphenolic compounds from red wine lees. Foods 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Screening the effect of four ultrasound-assisted extraction parameters on hesperidin and phenolic acid content of aqueous citrus pomace extracts. Food Biosci. 2018, 21, 20–26. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Boudhrioua, N.M.; Ghoul, M. Effect of different operating conditions on the extraction of phenolic compounds in orange peel. Food Bioprod. Proc. 2015, 96, 161–170. [Google Scholar] [CrossRef]
- Wang, J.; Ma, H.; Pan, Z.; Qu, W. Sonochemical effect of flat sweep frequency and pulsed ultrasound (FSFP) treatment on stability of phenolic acids in a model system. Ultrason. Sonochem. 2017, 39, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef]
- Ma, Y.-Q.; Chen, J.-C.; Liu, D.-H.; Ye, X.-Q. Simultaneous extraction of phenolic compounds of citrus peel extracts: Effect of ultrasound. Ultrason. Sonochem. 2009, 16, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ana, C.-C.; Jesús, P.-V.; Hugo, E.-A.; Teresa, A.-T.; Ulises, G.-C.; Neith, P. Antioxidant capacity and UPLC–PDA ESI–MS polyphenolic profile of Citrus aurantium extracts obtained by ultrasound assisted extraction. J. Food Sci. Technol. 2018, 55, 5106–5114. [Google Scholar] [CrossRef] [PubMed]
- Nishad, J.; Saha, S.; Kaur, C. Enzyme- and ultrasound-assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: A comparative study. J. Food Proc. Preserv. 2019, 43, e14046. [Google Scholar] [CrossRef]
- Dahmoune, F.; Boulekbache, L.; Moussi, K.; Aoun, O.; Spigno, G.; Madani, K. Valorization of Citrus limon residues for the recovery of antioxidants: Evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crops Prod. 2013, 50, 77–87. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; Guerra-Hernández, E.J.; Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; García-Villanova, B.; Verardo, V. Optimization of ultrasound-assisted extraction via sonotrode of phenolic compounds from orange by-products. Foods 2021, 10, 1120. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abd El Aziz, N.M.; Youssef, M.M.; El-Sohaimy, S.A. Optimization conditions of ultrasound-assisted extraction of phenolic compounds from orange peels using response surface methodology. J. Food Proc. Preserv. 2021, 45, e15870. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Pingret, D.; Fabiano-Tixier, A.-S.; Le Bourvellec, C.; Renard, C.M.; Chemat, F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing; Wiley: Weinheim, Gemangy, 2002. [Google Scholar]
- Afifi, S.M.; Kabbash, E.M.; Berger, R.G.; Krings, U.; Esatbeyoglu, T. Comparative untargeted metabolic profiling of different parts of Citrus sinensis fruits via liquid chromatography–mass spectrometry coupled with multivariate data analyses to unravel authenticity. Foods 2023, 12, 579. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J. Valorisation potential of Cabernet grape pomace for the recovery of polyphenols: Process intensification, optimisation and study of kinetics. Food Bioprod. Proc. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Kaltsa, O.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. The effect of ultrasonication pretreatment on the production of polyphenol-enriched extracts from Moringa oleifera L.(drumstick tree) using a novel bio-based deep eutectic solvent. Appl. Sci. 2019, 10, 220. [Google Scholar] [CrossRef]
- Alibante, A.; Lakka, A.; Bozinou, E.; Chatzilazarou, A.; Lalas, S.; Makris, D.P. Integrated green process for the extraction of red grape pomace antioxidant polyphenols using ultrasound-assisted pretreatment and β-cyclodextrin. Beverages 2021, 7, 59. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-cyclodextrin as a green co-solvent in the aqueous extraction of polyphenols from waste orange peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Abdoun, R.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P. Process optimization and stability of waste orange peel polyphenols in extracts obtained with organosolv thermal treatment using glycerol-based solvents. ChemEngineering 2022, 6, 35. [Google Scholar] [CrossRef]
- Gómez-Urios, C.; Viñas-Ospino, A.; Penadés-Soler, A.; Lopez-Malo, D.; Frígola, A.; Esteve, M.; Blesa, J. Natural deep eutectic solvents as main solvent for the extraction of total polyphenols of orange peel. Biol. Life Sci. Forum 2021, 6, 110. [Google Scholar] [CrossRef]
- Goulas, V.; Manganaris, G.A. Exploring the phytochemical content and the antioxidant potential of Citrus fruits grown in Cyprus. Food Chem. 2012, 131, 39–47. [Google Scholar] [CrossRef]
- Chen, X.-M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.K.; Sinha, P.; Emanuel, N.; Kumar, N.; Sami, R.; Khojah, E.; Al-Mushhin, A.A. Effect of ultrasound-assisted pretreatment on extraction efficiency of essential oil and bioactive compounds from citrus waste by-products. Separations 2021, 8, 244. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Sadaka, C.; Generalić Mekinić, I.; Garzoli, S.; Švarc-Gajić, J.; Rodrigues, F.; Morais, S.; Moreira, M.M.; Ferreira, E.; Spigno, G. The chemical variability, nutraceutical value, and food-industry and cosmetic applications of citrus plants: A critical Review. Antioxidants 2023, 12, 481. [Google Scholar] [CrossRef] [PubMed]







| Variable | Code | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ampl (%) | X1 | 60 | 70 | 80 |
| t (min) | X2 | 10 | 15 | 20 |
| Design Point | Process Variables | Response | ||
|---|---|---|---|---|
| YTP (mg CGAE g−1 DM) | ||||
| X1 (%Ampl) | X2 (t, min) | Measured | Predicted | |
| 1 | −1 (60) | −1 (10) | 11.70 | 11.65 |
| 2 | −1 (60) | 1 (20) | 12.74 | 12.76 |
| 3 | 1 (80) | −1 (10) | 12.76 | 12.62 |
| 4 | 1 (80) | 1 (20) | 12.67 | 12.62 |
| 5 | −1 (60) | 0 (15) | 12.35 | 12.37 |
| 6 | 1 (80) | 0 (15) | 12.64 | 12.78 |
| 7 | 0 (70) | −1 (10) | 12.02 | 12.17 |
| 8 | 0 (70) | 1 (20) | 12.69 | 12.72 |
| 9 | 0 (70) | 0 (15) | 12.70 | 12.61 |
| 10 | 0 (70) | 0 (15) | 12.65 | 12.61 |
| 11 | 0 (70) | 0 (15) | 12.75 | 12.61 |
| # | Compound | Yield (μg g−1 DM) | ||
|---|---|---|---|---|
| US | Stirring | US + Stirring | ||
| Phenolic acids | ||||
| 1 | Neochlorogenic acid | 458.88 ± 38.02 a | 476.72 ± 33.43 a | 534.33 ± 42.11 a |
| 2 | Chlorogenic acid | 699.64 ± 54.62 a | 734.80 ± 60.44 a,b | 818.02 ± 58.88 b |
| 3 | Caffeic acid | 120.14 ± 8.44 a | 122.06 ± 9.03 a | 131.51 ± 9.21 a |
| 4 | Ferulic acid | 145.18 ± 10.21 a | 154.97 ± 11.05 a,b | 170.53 ± 11.74 b |
| Total | 1423.84 | 1488.55 | 1654.40 | |
| Flavanones | ||||
| 5 | Narirutin | 1016.51 ± 73.11 a | 1094.86 ± 80.91 a,b | 1195.84 ± 75.44 b |
| 6 | Hesperidin | 1960.95 ± 112.04 a | 1984.14 ± 135.43 a | 2111.52 ± 123.02 a |
| Total | 2977.47 | 3079.00 | 3307.36 | |
| Flavones | ||||
| 7 | Didymin | 498.80 ± 30.30 a | 512.49 ± 36.54 a,b | 566.89 ± 29.98 b |
| 8 | Sinensetin | 145.13 ± 8.91 a | 148.22 ± 6.66 a | 162.01 ± 11.22 a |
| 9 | Nobiletin | 515.76 ± 28.54 a | 522.65 ± 40.12 a | 574.22 ± 13.56 b |
| 10 | Demethylnobiletin | 230.23 ± 12.69 a | 231.49 ± 12.24 a | 253.40 ± 9.71 a |
| Total | 1389.91 | 1414.84 | 1556.52 | |
| Sum | 5791.22 | 5982.39 | 6518.28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belsi, K.; Vogiatzi, X.; Palaiogiannis, D.; Chatzimitakos, T.; Lalas, S.I.; Makris, D.P. Ultrasonication-Assisted Aqueous Extraction of Waste Orange Peel Polyphenols: Optimization of Process Variables and Effect on Extract Composition. Compounds 2024, 4, 301-314. https://doi.org/10.3390/compounds4020016
Belsi K, Vogiatzi X, Palaiogiannis D, Chatzimitakos T, Lalas SI, Makris DP. Ultrasonication-Assisted Aqueous Extraction of Waste Orange Peel Polyphenols: Optimization of Process Variables and Effect on Extract Composition. Compounds. 2024; 4(2):301-314. https://doi.org/10.3390/compounds4020016
Chicago/Turabian StyleBelsi, Katiana, Xenia Vogiatzi, Dimitrios Palaiogiannis, Theodoros Chatzimitakos, Stavros I. Lalas, and Dimitris P. Makris. 2024. "Ultrasonication-Assisted Aqueous Extraction of Waste Orange Peel Polyphenols: Optimization of Process Variables and Effect on Extract Composition" Compounds 4, no. 2: 301-314. https://doi.org/10.3390/compounds4020016
APA StyleBelsi, K., Vogiatzi, X., Palaiogiannis, D., Chatzimitakos, T., Lalas, S. I., & Makris, D. P. (2024). Ultrasonication-Assisted Aqueous Extraction of Waste Orange Peel Polyphenols: Optimization of Process Variables and Effect on Extract Composition. Compounds, 4(2), 301-314. https://doi.org/10.3390/compounds4020016