(1E)-1,2-Diaryldiazene Derivatives Containing a Donor–π-Acceptor-Type Tolane Skeleton as Smectic Liquid–Crystalline Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Materials
2.3. Typical Synthetic Procedure for (1E)-1-(4-Cyanophenyl)-2-[4-[2-(4-decyloxyphenyl)ethynyl]phenyl]diazene (1a)
2.3.1. (1E)-1-(4-Cyanophenyl)-2-[4-[2-(4-decyloxyphenyl)ethynyl]phenyl]diazene (1a)
2.3.2. (1E)-1-(4-Cyanophenyl)-2-[4-[2-(4-(7,7,8,8,9,9,10,10,10-nonafluorodecyloxy)phenyl)ethynyl]phenyl]diazene (1b)
2.3.3. (1E)-1-(4-Cyanophenyl)-2-[4-[2-(4-(5,5,6,6,7,7,8,8,9,9,10,10,10-tridecafluorodecyloxy)phenyl)ethynyl]phenyl]diazene (1c)
2.4. Phase Transition Properties
2.5. Photophysical Properties
2.6. Theoretical Assessment
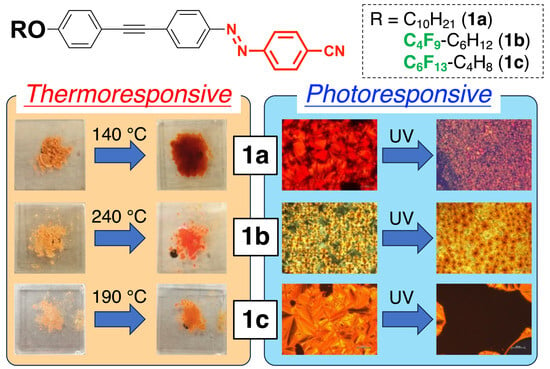
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, C.; Lai, L.; Li, P.; Wu, Z.; Cheng, W.; Yin, M. Organic dye assemblies with aggregation-induced photophysical changes and their bio-applications. Aggregate 2021, 2, e39. [Google Scholar] [CrossRef]
- Sun, N.; Cai, Y.; Cui, T.; Yang, W.; Hu, Y. Organic dye fluorescent fibers for wearable devices. Opt. Mater. 2023, 143, 114216. [Google Scholar] [CrossRef]
- Arka, G.N.; Prasad, S.B.; Singh, S. Comprehensive study on dye sensitized solar cell in subsystem level to excel performance potential: A review. Sol. Energy 2021, 226, 192–213. [Google Scholar] [CrossRef]
- Prajapat, K.; Dhonde, M.; Sahu, K.; Bhojane, P.; Murty, V.V.S.; Shirage, P.M. The evolution of organic materials for efficient dye-sensitized solar cells. J. Photochem. Photobiol. C 2023, 55, 100586. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Zhao, H.; Li, B.; Hamel, J.; Yang, Y.; Cao, D.; Natan, A.; Zhu, H. Abundant organic dye as an anolyte for aqueous flow battery with multielectron transfer. ACS Appl. Energy Mater. 2018, 2, 7425–7437. [Google Scholar] [CrossRef]
- Mustroph, H.; Stollenwerk, M.; Bressau, V. Current developments in optical data storage with organic dyes. Angew. Chem. Int. Ed. 2006, 45, 2016–2035. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.; Ahmad, S.; Atiq, A.; Farooq, M.A.; Huang, M. Recent progress in azobenzene-based supramolecular materials and applications. Chem. Rec. 2023, 23, e202300126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Feng, Y.; Fent, W. Azobenzene-based solar thermal fuels: A review. Nano-Micro Lett. 2022, 14, 138. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Höglsperger, F.; Vos, B.E.; Hofemeier, A.D.; Seyfried, M.D.; Stövesand, B.; Alavizargar, A.; Topp, L.; Heuer, A.; Betz, T.; Ravoo, B.J. Rapid and reversible optical switching of cell membrane area by an amphiphilic azobenzene. Nat. Commun. 2023, 14, 3760. [Google Scholar] [CrossRef]
- Derkowska-Zielinska, B.; Skowronski, L.; Sypniewska, M.; Chomicki, D.; Smokal, V.; Kharchenko, O.; Naparty, M.; Krupka, O. Functionalized polymers with strong push-pull azo chromophores in side chain for optical application. Opt. Mater. 2018, 85, 391–398. [Google Scholar] [CrossRef]
- Ikeda, T.; Tsutsumi, O. Optical switching and image storage by means of azobenzene liquid-crystal films. Science 1995, 268, 1873–1875. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, O.; Shiono, T.; Ikeda, T.; Galli, G. Photochemical phase transition behavior of nematic liquid crystals with azobenzene moieties as both mesogens and photosensitive chromophores. J. Phys. Chem. B 1997, 101, 1332–1337. [Google Scholar] [CrossRef]
- Tsutsumi, O.; Kitsunai, T.; Kanazawa, A.; Shiono, T.; Ikeda, T. Photochemical phase transition behavior of polymer azobenzene liquid crystals with electron-donating and -accepting substituents at the 4,4′-positions. Macromolecules 1998, 31, 355–359. [Google Scholar] [CrossRef]
- Okano, K.; Shishido, A.; Ikeda, T. Photochemical phase transition behavior of highly birefringent azotolane liquid-crystalline polymer films: Effects of the position of the tolane group and the donor–acceptor substituent in the mesogen. Macromolecules 2006, 39, 145–152. [Google Scholar] [CrossRef]
- Okano, K.; Tsutsumi, O.; Shishido, A.; Ikeda, T. Azotolane liquid-crystalline polymers: Huge change in birefringence by photoinduced alignment change. J. Am. Chem. Soc. 2006, 128, 15368–15369. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Konno, T. Development of donor-π-acceptor-type fluorinated tolanes as compact condensed phase luminophores and applications in photoluminescent liquid-crystalline molecules. Chem. Rec. 2023, 23, e202300094. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Yoshida, K.; Hara, M.; Agou, T.; Yasui, T.; Konno, T. Effects of fluorine atoms introduced into flexible chains or mesogenic structures on their liquid-crystalline and photoluminescence characteristics. J. Mol. Liq. 2024, 393, 123545. [Google Scholar] [CrossRef]
- Yamada, S.; Yoshida, K.; Kataoka, M.; Hara, M.; Konno, T. Donor-π-acceptor-type fluorinated tolane containing a semifluoroalkoxy chain as a condensed-phase luminophore. Molecules 2023, 28, 2764. [Google Scholar] [CrossRef]
- Yamada, S.; Yoshida, K.; Sakurai, T.; Hara, M.; Konno, T. Effect of fluorine atoms in flexible chains on the phase transitions and photophysical behavior of D-π-A-type 4-alkoxy-4′-cyanophenylacetylene. Mol. Syst. Des. Eng. 2022, 7, 720–724. [Google Scholar] [CrossRef]
- Takeda, Y.; Okumura, S.; Minakata, S. Oxidative dimerization of aromatic amines using tBuOI: Entry to unsymmetric aromatic azo compounds. Angew. Chem. Int. Ed. 2012, 51, 7804–7808. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Lin, C.; Takeda, Y.; Minakata, S. Oxidative dimerization of (hetero)aromatic amines utilizing t-BuOI leading to (hetero)aromatic azo compounds: Scope and mechanistic studies. J. Org. Chem. 2013, 78, 12090–12105. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Li, M.; Reimers, J.R.; Ford, M.J.; Kobayashi, R.; Amos, R.D. Accurate prediction of the properties of materials using the CAM-B3LYP density functional. J. Comput. Chem. 2021, 42, 1486–1497. [Google Scholar] [CrossRef]
- Li, H.; Jensen, J.H. Improving the efficiency and convergence of geometry optimization with the polarizable continuum model: New energy gradients and molecular surface tessellation. J. Comput. Chem. 2004, 25, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, Y.; Murakoshi, T.; Hishida, M.; Saito, K. Examination of molecular packing in orthogonal smectic liquid crystal phases: A guide for molecular design of functional smectic phases. Phys. Chem. Chem. Phys. 2017, 19, 25518–25526. [Google Scholar] [CrossRef]
- Merino, E.; Ribagorda, M. Control over molecular motion using the cis–trans photoisomerization of the azo group. Beilstein J. Org. Chem. 2012, 8, 1071–1090. [Google Scholar] [CrossRef] [PubMed]
- Tiberio, G.; Muccioli, L.; Berardi, R.; Zannoni, C. How does the trans–cis photoisomerization of azobenzene take place in organic solvents? ChemPhysChem 2010, 11, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Würthner, F. Supramolecularly engineered J-aggregates based on perylene bisimide dyes. Acc. Chem. Res. 2021, 54, 642–653. [Google Scholar] [CrossRef]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-aggregates: From serendipitous discovery to supramolecular engineering of functional dye materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef]






| Compound | Phase Sequence and Phase Transition Temperature [°C] (Enthalpy [kJ mol−1]) | |
|---|---|---|
| 1a | [H] | Cr 71 (−9.5) Cr 97 (9.1) Cr 107 (8.1) SmE 160 (4.9) SmA 228 (−) 2 Iso |
| [C] | Cr 23 (−0.64) Cr 62 (−4.2) SmE 155 (−6.2) SmA 225 (−) 2 Iso | |
| 1b | [H] | Cr 132 (2.2) SmE 205 (6.6) SmA 285 (7.2) Iso |
| [C] | Cr 124 (−1.9) SmE 201 (−5.3) SmA 284 (−5.0) Iso | |
| 1c | [H] | Cr 57 (−2.2) Cr 107 (8.6) Cr 126 (1.6) Cr 176 (10.9) Sm 225 (−) 2 Iso |
| [C] | G 81 Cr 138 (−10.3) Sm 223 (−) 2 Iso | |
| 1a | 1b | ||||
|---|---|---|---|---|---|
| Label | 2θ [°]/d Spacing [nm] | hkl | Label | 2θ [°]/d Spacing [nm] | hkl |
| (180 °C) | (250 °C) | ||||
| 1 | 2.17/4.07 | 001 | 1 | 2.22/3.97 | 001 |
| (100 °C) | 2 | 4.38/2.01 | 002 | ||
| 2 | 2.34/3.77 | 001 | (150 °C) | ||
| 3 | 4.68/1.89 | 002 | 3 | 2.18/4.05 | 001 |
| 4 | 7.04/1.25 | 003 | 4 | 4.42/2.00 | 002 |
| 5 | 20.44/0.43 | 110 | 5 | 20.14/0.44 | 110 |
| 6 | 22.28/0.40 | 200 | 6 | 22.00/0.40 | 200 |
| 7 | 28.23/0.32 | 210 | 7 | 27.83/0.32 | 210 |
| Compound | λ [nm] 1 (ε [103, L mol−1 cm−1]) | λcalcd [nm] 2 (Oscillator Strength) | HOMO/LUMO [eV] 2 (∆EH-L) | Theoretical Transition 2 (Contribution) |
|---|---|---|---|---|
| 1a | 294 (18.6), 384 (24.3) | 384 (f = 1.98) | −7.22 eV/−2.08 eV (5.13 eV) | HOMO → LUMO (78%) HOMO−1 → LUMO (14%) HOMO → LUMO+1 (4%) |
| 1b | 377 (18.5) | 384 (f = 1.98) | −7.23 eV/−2.09 eV (5.14 eV) | HOMO → LUMO (78%) HOMO−1 → LUMO (14%) HOMO → LUMO+1 (4%) |
| 1c | 293 (20.7), 351 (18.2) | 383 (f = 1.98) | −7.25 eV/−2.09 eV (5.16 eV) | HOMO → LUMO (79%) HOMO−1 → LUMO (14%) HOMO → LUMO+1 (4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Yoshida, K.; Eguchi, Y.; Hara, M.; Yasui, M.; Konno, T. (1E)-1,2-Diaryldiazene Derivatives Containing a Donor–π-Acceptor-Type Tolane Skeleton as Smectic Liquid–Crystalline Dyes. Compounds 2024, 4, 288-300. https://doi.org/10.3390/compounds4020015
Yamada S, Yoshida K, Eguchi Y, Hara M, Yasui M, Konno T. (1E)-1,2-Diaryldiazene Derivatives Containing a Donor–π-Acceptor-Type Tolane Skeleton as Smectic Liquid–Crystalline Dyes. Compounds. 2024; 4(2):288-300. https://doi.org/10.3390/compounds4020015
Chicago/Turabian StyleYamada, Shigeyuki, Keigo Yoshida, Yuto Eguchi, Mitsuo Hara, Motohiro Yasui, and Tsutomu Konno. 2024. "(1E)-1,2-Diaryldiazene Derivatives Containing a Donor–π-Acceptor-Type Tolane Skeleton as Smectic Liquid–Crystalline Dyes" Compounds 4, no. 2: 288-300. https://doi.org/10.3390/compounds4020015
APA StyleYamada, S., Yoshida, K., Eguchi, Y., Hara, M., Yasui, M., & Konno, T. (2024). (1E)-1,2-Diaryldiazene Derivatives Containing a Donor–π-Acceptor-Type Tolane Skeleton as Smectic Liquid–Crystalline Dyes. Compounds, 4(2), 288-300. https://doi.org/10.3390/compounds4020015