1. Introduction
Carbon-based nanodots and nanoparticles have been recognized as promising functional materials due to their excellent colloidal stability, biocompatibility, possibility of chemical modification, and peculiar optical properties [
1]. One of the most striking features of the carbonaceous nanodots and nanoparticles is their fluorescence typically excited by UV or visible-range irradiation. The fluorescence properties can be used in light-emitting devices, including screens for portable devices, yet this technology has not been matured. Application of carbon-based nanoparticles in qualitative sensing and the quantitative determination of heavy metals and organic species (exploiting the sensitivity of their emission properties to the presence of various analytes) has been better explored. Other applications of the carbonaceous nanoparticles include catalysis and energy harvesting [
1]. These applications also rely on the optical properties of the nanoparticles: the ability to absorb UV and visible light, being converted in the excited state and capable of initiating photoreactions. The essence of the use of dispersed (colloidal) nanoparticles is due to two factors. The emission of the nanoparticles due to the defects in the graphitic core is, as a rule, strongly dependent on the size of the core (hence, such objects are usually called carbon nanodots). The effects due to the surface functional groups (optical properties, binding capacity, catalytic activity, etc.) are also more pronounced for colloidal particles due to the increased specific surface area. Moreover, nanosized particles are more stable against sedimentation, allowing their prolonged use in aqueous media. Nitrogen-doped nanoparticles are beneficial over other carbon-based nanomaterials due to the higher quantum yield and the possibility of fluorescence excitation by light with a wavelength of about 500 nm [
2], which is close to the window of biological tissues transparency, enabling the application of such carbon nanoparticles for in vivo imaging and phototherapy [
1].
Microwave or solvothermal treatment of a mixture of citric acid and urea are simple and efficient approaches to prepare nitrogen-doped carbon nanodots and nanoparticles [
3]. These processes can be performed in bulk as well as in a solution in water or other polar solvents such as ethanol or dimethylformamide [
4]. The attempts to elucidate the products’ structure have been recently reported [
5,
6]. It has been argued that the primary interaction of urea and citric acid leads to citrazinic acid, described in 1893 [
7], whereas further condensation reactions yield well-defined low-molecular species such as 4-hydroxy-1
H-pyrrolo [3,4-
c]pyridine-1,3,6(2
H,5
H)-trione [
6] along with more complex products mixtures including oligomeric ureas, melamine, guanidine, and other CN ring structures stabilized by the oligomers and forming sophisticated hydrogen bond networks which stabilize the nanoparticles [
5].
Although certain progress has been made towards the investigation of the molecular mechanism of the nanoparticle formation from urea and citric acid [
8], tuning their optical and other properties has largely remained an empirical issue due to complexity of the process. Therefore, many reports have appeared discussing the effects of the process duration, temperature, and the components ratio on the synthesis outcome. For example, the microwave-assisted (domestic microwave oven) formation of carbon nanodots in an aqueous solution of a 1:1 mixture of urea with citric acid, the synthesis duration being 30 to 300 s, has been investigated in [
9]. Gradual change in the product coloration has revealed the reaction progress. The absorption spectra have been qualitatively similar when the treatment duration has been of 60–300 s, its intensity being regularly increased with the treatment duration. The fluorescence (excitation wavelength 300–350 nm, emission band maximum 435–440 nm) has been observed only for the samples treated during 150–300 s. The spectral shape as well as the band gap have been found independent of the synthesis duration, meaning that the nature of the emitting species has not been changed throughout the process. It should be noticed that the dialysis of the samples to remove the low-molecular products has not been mentioned by the authors.
In a similar study [
10], the effect of the heating rate (1.6–9.6 deg/min) and duration (up to 90 min) on the outcome of the hydrothermal treatment of a urea–citric acid mixture has been studied. The urea/citric acid mass ratio was constant (18.5:1), whereas the total components concentration was varied between 0.025 and 1.27 mol/L. The product photoluminescence intensity was passed through a maximum at the process duration corresponding to a temperature of 150–170 °C; further weakening of the photoluminescence has been ascribed to the formation of larger nanoparticles. Indeed, the mean size of the particles formed at 150 and 180 °C at the lowest heating rate was 2 and 29 nm, respectively. No effect of the heating rate on the quantum yield of fluorescence (31–33%) for the product obtained at the optimal duration has been found. Hence, it has been concluded that the synthesis temperature is the most important factor determining the formation of stable, highly fluorescent nitrogen-doped carbon dots. Unfortunately, the details of the sample purification upon the synthesis have not been mentioned in this report either.
Another study of a similar hydrothermal process (temperature 140–240 °C, duration 2–10 h, urea/citric acid mass ratio 1:223 to 1:22, aqueous solution) [
11] has led to a somewhat different conclusion. Upon the synthesis, the product was filtered and subject to dialysis in order to remove large particles and low-molecular species. In this study, the strongest fluorescence was observed in the case of the longest treatment at the highest temperature (the 5–10 nm graphitic particles were found in the product by means of TEM), which is in seeming contradiction with the findings in [
10]. However, the initial mixture compositions in these studies were strongly different: a large excess of urea in [
10] and of citric acid in [
11]. Therefore, the composition of the reaction mixture is another important factor affecting the process outcome.
The influence of the mixture composition has been addressed in several studies, including [
12] (microwave treatment of an aqueous solution), [
13] (microwave treatment of a humid mixture), and [
14] (solvothermal treatment of ferric citrate and urea in dimethylformamide). The strong dependence of the products’ optical properties on the mixture composition has been revealed in these studies, yet the details have been different. In the case of the microwave-assisted hydrothermal treatment [
12], the urea/citric acid mass ratios between 5:1 and 1:5 were examined, with the aqueous solution concentration being 13%. The products were purified via centrifugation and dialysis. Electronic absorption spectra of the products contained several bands with maximums at about 275, 330, and 415 nm, with their ratio being variable. Emission spectra of the products obtained in the excess of citric acid showed blue fluorescence, with the maximum at about 440 nm (excitation wavelength 370 nm), whereas those obtained at the 1:1 ratio or in excess of urea revealed green fluorescence (emission maximum at 540 nm) when excited at 408.5 nm. The obtained products were used to detect allantoin: the nanodots obtained in the excess of citric acid revealed the enhancement of the fluorescence in the presence of allantoin, which has been ascribed to the hydrogen bonding between allantoin and surface citrazinic acid groups of the nanodots, as supported by the XPS data. Moreover, the graphitic structure of the nanodots has been confirmed by TEM. The products obtained in the excess of urea have been found insensitive to the presence of allantoin.
When the microwave-assisted synthesis was performed with only small amount of water (10 g per 100 g of the solids mixture; the urea/citric acid mass ratio was varied between 3:1 and 1:3) [
13], the absorption spectra were slightly different. The samples obtained in the excess of citric acid revealed the bands at about 350 and 450 nm (the long-wave band has appeared as a shoulder shifting to a shorter wavelength with the decrease in citric acid excess), whereas the bands were at about 330 and 415 nm at the 1:1 ratio or in excess of urea, with the long-wave band being stronger. The samples revealed a blue (excess of citric acid) or green (1:1 or excess of urea) fluorescence under 360 nm UV irradiation. When the excitation wavelength was tuned (340–450 nm), the emission band shifted from 410 to 550 nm (excess of citric acid) and from 370 to 550 nm (excess of urea). Longer excitation wavelengths have not been probed. It should be noted that the dialysis stage has not been mentioned in this study.
In the case of the solvothermal treatment in dimethylformamide (160 °C, 6 h) [
14], ferric citrate was utilized instead of citric acid as the precursor, and the urea/ferric citrate mass ratio was varied between 1:1 and 6:1 (the compositions in excess of citrate have not been studied). Only centrifugation has been mentioned as the product purification method. The electronic absorption spectrum of the nanodots contained an n-π* transition band at 340 nm and a shoulder at 420 nm. The luminescence spectra showed a blue emission band at about 450 nm (excitation at 300–370 nm), blue and green emission bands when excited at 380 nm, and a green emission band at about 530 nm at longer excitation wavelengths (390–510 nm). This pattern was found similar independently of the reaction mixture composition. It has not been mentioned if iron has been retained in the nanodots or in the external liquid phase.
Hence, to the best of our knowledge, studies of the effect of the precursors mixture composition on the outcome of solvothermal treatment of urea and citric acid in organic media have been lacking. However, it is known that the change of solvent during the heat treatment can strongly affect the optical properties of the product. For example, the heating of a 1:2 urea–citric acid mixture (180 °C, 10 h) in water, ethanol, and dimethylformamide has resulted in blue (emission maximum at 450 nm), yellow (550 nm), and red-emitting (650 nm) carbon nanodots, respectively [
4]. The possibility of increasing both the excitation and emission wavelengths is interesting in view of the solar energy harvesting and in vivo biomedical applications. Therefore, in the present study, we decided to probe the effect of the urea–citric acid mixture composition on the outcome of its solvothermal treatment in dimethylformamide. The range of compositions was chosen to cover both urea- and citric-acid-rich mixtures (7:1 to 1:7). Although the temperature and duration of the synthesis could likely affect product properties as well, we kept them constant in the scope of this study.
Furthermore, it has been argued that the codoping of carbon nanoparticles with nitrogen and sulfur can be advantageous in view of their antioxidant properties [
15] and the detection of nitric oxide [
16]. The codoping has been achieved in the mentioned studies via the introduction of either α-lipoic acid [
15] or sodium thiosulfate [
16] into a urea–citric acid mixture during the hydrothermal treatment. Several studies have mentioned the possibility of direct introduction of nitrogen and sulfur into carbon nanodots via hydrothermal treatment of a mixture of citric acid and thiourea [
17,
18,
19,
20,
21]. To the best of our knowledge, only two reports have discussed the solvothermal treatment of thiourea–citric acid mixtures in dimethylformamide. It has been marked that the obtained nanoparticles can be self-oxidized during storage, which affects their optical properties [
22]. In the other study [
23], several peculiar pH- and solvent-dependent effects have been described as well as the possibility to use the prepared nanoparticles for the detection of complex organic molecules, but the influence of the precursors mixture composition has not been detailed. Therefore, we also explored the solvothermal treatment of thiourea–citric acid mixtures of different composition in dimethylformamide and compared its outcome with the urea-based mixtures.
In our study, we mainly addressed the absorbance and fluorescence of the prepared products as well as their fluorescence sensitivity to the presence of mercury(II) ions. We have recently shown [
24] that the so-prepared carbonaceous particles exhibit the selective quenching of the fluorescence by this heavy metal; therefore, it was of additional interest to probe the effect of the initial mixture composition on the colloidal product sensitivity. The major novelty of our research in comparison with the earlier reports consists of the comprehensive investigation of the optical properties of colloidal products obtained via the solvothermal treatment of urea or thiourea with citric acid, the mixture composition being widely varied between 7:1 and 1:7 (by weight) and the other synthesis conditions being identical; moreover, we compared the properties of the urea- and thiourea-based products obtained under identical conditions.
4. Discussion
We considered a wide range of compositions of urea–citric acid mixtures subject to solvothermal treatment in this study. Since the properties of the so-obtained nanoparticles were expected to strongly depend on the nature of the solvent used in the synthesis [
4], we compared our results with the available reports on relevant solvothermal preparations in dimethylformamide [
4,
14,
26,
27,
28]. In general, our findings coincided with the properties of carbon nanodots prepared in other studies in excess of urea or at a 1:1 mass ratio of the precursors [
4,
14,
26,
28] as well as in slight excess of citric acid [
4,
27]: the multicolor excitation-dependent fluorescence of the solvothermal treatment products was observed. Important progress made in our study was the direct examination of the synthesized nanoparticles by means of the NTA technique, without isolation for TEM study. Comparison of the samples prepared over a wide range of the mixtures composition, 7:1 to 1:7, revealed that the highest yield of the colloidal particles was achieved at the urea/citric acid mass ratio close to unity (
Table 1), whereas their concentration in the product rapidly decreased at more than threefold excess of either precursor. Moreover, the characteristic groups of the samples were revealed based on the absorption (
Figure 4a) and emission spectra (
Figure 6a and
Figure 8): those prepared in significant excess of urea (7:1 to 3:1), at equal mass ratio or in slight excess of citric acid (1:1 and 1:3), and at large excess of citric acid (1:5 and 1:7). Four types of fluorophores differing in the excitation wavelength and the emission spectrum were detected in the colloidal products (
Figure 8). Their presence and fractions in the sample depended on the precursors ratio during the synthesis, as described in detail in the
Section 3. Hence, variation of the initial precursor ratio during the solvothermal treatment is a facile and powerful tool to tune the optical properties of the obtained products, in addition to variation of other process conditions such as temperature and the duration of the treatment [
27] or the solvent nature [
4].
Comparison of optical properties of the purified colloidal products and the dialyzed low-molecular species revealed that they were strongly different. Hence, the colloidal particles were not merely associated low-molecular dyes or inert nanoparticles bearing dye-like surface groups; the nature of the nanoparticle core affected the electronic structure of the fluorophores.
It should be noted that the yield of colloidal particles estimated from their concentration assessed by means of the NTA was quite low (
Table 1). That fact could be caused by at least two reasons. First, the gaseous products and low-molecular species separated off by dialysis could indeed constitute the major fraction of the mixture under the probed reaction conditions (heating at 160 °C during 8 h). In that case, the yield of colloidal particles could be improved by tuning the reaction conditions, for example using a factorial-based approach [
27]. Second, significant part of the prepared samples could be constituted by ultrasmall nanodots with size below 10 nm. This size is at the edge of the sensitivity of the scattering-based NTA instrument, and the corresponding particles could be counted inaccurately (although a small fraction of 7–15 nm particles was detected in the sample). To verify that suggestion, we plan to perform comprehensive particle size analysis by means of the NTA, TEM, AFM, and DLS in the future study. Another possible reason for the low yield of the particles, the formation of a large amount of insoluble products, was ruled out in the present study, since we did not observe any noticeable precipitate in the samples, irrespective of the composition. Let us notice that when the precursor concentration of the treatment temperature was increased (the data on these samples will be reported elsewhere), the precipitate was indeed formed during the synthesis.
Another feature of the samples prepared by us was the relatively low quantum yield of fluorescence estimated in our study (1–4%,
Table 2). The relevant earlier studies have reported the quantum yield of 15 [
26,
27], 39–43% [
28], and up to 48% [
14]. Although the upper edge of the reported values seems unusually high (let us notice that the latter two reports stating the highest quantum yield have not mentioned the dialysis step of the samples purification, and the obtained products could be contaminated with low-molecular impurities), it was reasonable to assume that typical quantum yield of the urea–citric acid nanodots should be 15%. The unpleasantly low quantum yield determined in our study was caused by two different reasons, depending on the precursor mixture composition. In the case of the urea-rich products (7:1–3:1), the major reason was the fact that the strongest emission was excited at 540–550 nm (
Figure 8), whereas we determined the quantum yield at the excitation wavelength 365 nm, with the corresponding band being relatively weak. From the shapes of the absorption and emission spectra of these samples, it is reasonable to assume that the quantum yield of fluorescence of the major emission band should be several times higher, but accurate data are not available at the moment. For the citric acid-rich samples (1:5 and 1:7), excitation at 365 nm corresponded to the major emission band (
Figure 8). However, despite the excellent colloidal stability during storage observed for those samples, they contained a fraction of larger particles separable by centrifugation at 13k rpm during 30 min. Our preliminary results revealed that the emission intensity of the samples was strongly improved upon centrifugation. Therefore, it can be expected that further purification via centrifugation and/or fractional precipitation can noticeably improve the quantum yield observed for those samples. Finally, fluorescence of the colloidal products is pH-sensitive [
27], and further enhancement of the quantum yield can be expected in basic media instead of the neutral solutions used in the present study.
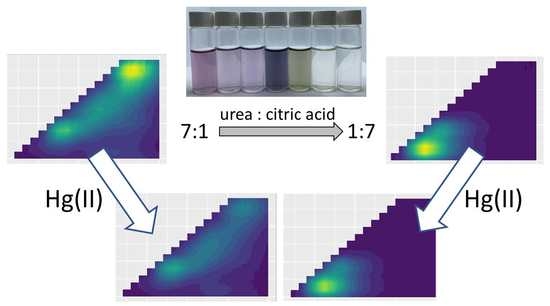
As shown in
Figure 10 and
Figure 11b, the composition of the precursor mixture affected the sensitivity of the obtained colloidal products to the presence of Hg(II) ions. The best results were obtained for the urea-rich samples (7:1–1:1), for which the detection limit was on the order of 0.1 µmol/L, whereas the citric-acid-rich ones revealed noticeable quenching of fluorescence at a Hg(II) concentration as high as 10 µmol/L. Even without the specific optimization of the detection conditions, the detection limit estimated in the present study was comparable to that reported elsewhere (0.06 µmol/L [
29], 0.05 µmol/L [
30]). It has been shown that the proper adjustment of the experimental conditions (for example, the concentration of the nanodots and pH [
31]) can improve the detection limit by 1–2 orders of magnitude. Indeed, our trials demonstrated that changing the medium to a borate-buffered solution enhanced the quenching of the fluorescence of the obtained colloidal products excited at 365 nm. The effect of the medium on the excitation wavelength-dependent sensitivity and the selectivity to the presence of different heavy metals is still to be explored.
The effect of the different sensitivities of the emission bands to Hg(II) depending on the excitation wavelength (
Figure 11a) has also been earlier reported for Cu(II) [
28]. It is expected that the proper choice of the excitation wavelength can be used to decrease the detection limit to very low concentrations, but the simultaneous measurement of other less sensitive bands can extend the detection range. Moreover, it is very likely that different species can exhibit different affinities to the surface sites of carbon nanodots, giving rise to the emission bands at different wavelengths. If so, the simultaneous detection of several heavy metals using a single probe can be achieved. Another peculiar issue is the effect of the colloidal fraction on the quenching-based determination of metal ions. On the one hand, our study indicated that the relatively large colloidal particles reduce the fluorescence intensity and quantum yield; however, they can also increase the local concentration of the metals due to adsorption, and this effect can be beneficial for analytical purposes.
The optical properties of the colloidal products prepared from the thiourea–citric acid solutions in dimethylformamide appeared less dependent on the precursor mixture composition. In comparison with more diverse urea-based systems, only two groups of qualitatively different samples were revealed. Those prepared in excess of citric acid (1:3 and 1:5) exhibited no absorption bands in the visible-range region (
Figure 4b), and only UV-induced fluorescence was observed (
Figure 10). The samples prepared in excess of urea or at equal mass fractions of the precursors (7:1–1:1) exhibited an additional absorption band, with a maximum at about 550 nm (
Figure 4b) and additional emission bands excited at 450 and 540 nm (
Figure 10). Irrespective of the precursor ratio, the UV-excited band was the strongest, with the relative intensity of the additional bands being the highest at the thiourea–citric acid ratio of 3:1. In contrast to the urea-based systems, the position of the UV-induced band (excitation at 340 nm, emission at 440 nm) was identical for the 7:1–1:5 samples. The yield of the colloidal products was lower than in the urea-based systems, the sensitivity to Hg(II) ions was weaker, and the colloidal stability of those products during storage was poorer. Less diverse set of products (i.e., more selective reactions occurring during the synthesis) can indicate the fact that higher temperature and/or longer duration of solvothermal treatment is demanded in the case of the thiourea-based systems in comparison with the urea-based ones. The earlier reported possibility of redox and acid-base transformations involving the products of thiourea–citric acid thermolysis [
22,
23] make them worth further comprehensive investigation.