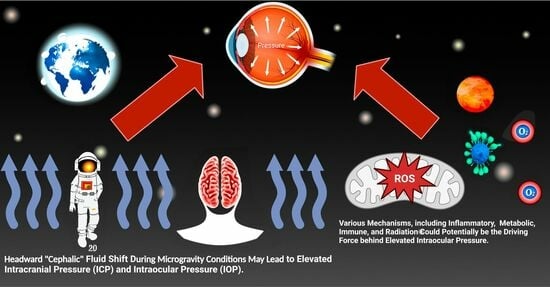
Intraocular Pressure during Spaceflight and Risk of Glaucomatous Damage in Prolonged Microgravity
Definition
:1. Introduction
2. Spaceflight-Associated Neuro-Ocular Syndrome
3. The Translaminar Pressure Gradient
- Relationship between ICP and retrolaminar tissue pressure: Extensive research has been conducted on the correlation between ICP and glaucoma [30]. Low ICP has been found to be associated with glaucoma, but the relationship between ICP and retrolaminar tissue pressure is complex [30]. Factors such as the size of the optic canal, thickness of the lamina cribrosa (a sieve-like structure in the optic nerve), and lymphatic outflow from the optic nerve can affect this relationship [30]. The lymphatic system also plays a crucial role in fluid drainage and maintaining tissue homeostasis. Alterations in lymphatic outflow from the optic nerve can affect the distribution of fluids and pressure within the retrolaminar region, potentially influencing the optic nerve’s health [30].
- Diurnal and positional variation on TLPG: The TLPG can vary throughout the day and in different body positions. Changes in posture and intra-abdominal pressure can influence ICP and, consequently, the TLPG [33]. It is important to consider these variations in TLPG when studying the potential impact of long-duration spaceflight on glaucoma development or progression. The precise impact of the circadian rhythm of IOP on the progression of glaucoma is yet to be definitively established. However, theories suggest the modulation of the TLPG as a contributing factor. Investigating the intricate interplay between circadian rhythms, fluctuations in IOP, and variations in TLPG holds the potential for gaining valuable insights into the development of preventive strategies aimed at preserving the optic nerve from progressive glaucomatous damage.
4. Increasing IOP as a Potential Mitigation Strategy for SANS
5. Potential Development of Glaucoma in Astronauts during Spaceflight
5.1. Mechanical and Structural Findings in SANS
Mechanical Factors
5.2. Vascular Factors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mader, T.H.; Gibson, C.R.; Pass, A.F.; Kramer, L.A.; Lee, A.G.; Fogarty, J.; Tarver, W.J.; Dervay, J.P.; Hamilton, D.R.; Sargsyan, A.; et al. Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-Duration Space Flight. Ophthalmology 2011, 118, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef]
- Wojcik, P.; Kini, A.; Al Othman, B.; Galdamez, L.A.; Lee, A.G. Spaceflight Associated Neuro-Ocular Syndrome. Curr. Opin. Neurol. 2020, 33, 62. [Google Scholar] [CrossRef] [PubMed]
- Mars, K. What Is Spaceflight Associated Neuro-Ocular Syndrome? Available online: http://www.nasa.gov/image-feature/what-is-spaceflight-associated-neuro-ocular-syndrome (accessed on 7 July 2023).
- Ong, J.; Mader, T.H.; Gibson, C.R.; Mason, S.S.; Lee, A.G. Spaceflight Associated Neuro-Ocular Syndrome (SANS): An Update on Potential Microgravity-Based Pathophysiology and Mitigation Development. Eye 2023, 37, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, E.; Ong, J.; Masalkhi, M.; Lee, A.G. Optic Neuropathy in Spaceflight-Associated Neuro-Ocular Syndrome (SANS). Ir. J. Med. Sci. 2023, 191, 2229–2230. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.R.; Ramachandran, V.; Khalid, R.; Keith Manuel, F.; Knowles, J.R.; Jones, J.A. Increased Intraocular Pressure in Glaucomatous, Ocular Hypertensive, and Normotensive Space Shuttle Crew. Aerosp. Med. Hum. Perform. 2021, 92, 728–733. [Google Scholar] [CrossRef]
- Huang, A.S.; Stenger, M.B.; Macias, B.R. Gravitational Influence on Intraocular Pressure. J. Glaucoma 2019, 28, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Mader, T.H.; Gibson, C.R.; Caputo, M.; Hunter, N.; Taylor, G.; Charles, J.; Meehan, R.T. Intraocular Pressure and Retinal Vascular Changes during Transient Exposure to Microgravity. Am. J. Ophthalmol. 1993, 115, 347–350. [Google Scholar] [CrossRef]
- Draeger, J.; Schwartz, R.; Groenhoff, S.; Stern, C. Self-Tonometry under Microgravity Conditions. Aviat. Space Env. Med. 1995, 66, 568–570. [Google Scholar] [CrossRef]
- Draeger, J.; Schwartz, R.; Groenhoff, S.; Stern, C. Self tonometry during the German 1993 Spacelab D2 mission. Ophthalmologe 1994, 91, 697–699. [Google Scholar]
- Nelson, E.S.; Mulugeta, L.; Myers, J.G. Microgravity-Induced Fluid Shift and Ophthalmic Changes. Life 2014, 4, 621–665. [Google Scholar] [CrossRef] [PubMed]
- Galdamez, L.A.; Brunstetter, T.J.; Lee, A.G.; Tarver, W.J. Origins of Cerebral Edema: Implications for Spaceflight-Associated Neuro-Ocular Syndrome. J. Neuroophthalmol. 2020, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, E.; Ong, J.; Lee, A.G. Factors Associated with Optic Disc Edema Development during Spaceflight. JAMA Ophthalmol. 2023, 141, 409. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Freddo, T.F.; Civan, M.; Gong, H. Aqueous Humor and the Dynamics of Its Flow: Mechanisms and Routes of Aqueous Humor Drainage. In Albert and Jakobiec’s Principles and Practice of Ophthalmology; Albert, D.M., Miller, J.W., Azar, D.T., Young, L.H., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1989–2033. ISBN 978-3-030-42634-7. [Google Scholar]
- Laurie, S.S.; Vizzeri, G.; Taibbi, G.; Ferguson, C.R.; Hu, X.; Lee, S.M.C.; Ploutz-Snyder, R.; Smith, S.M.; Zwart, S.R.; Stenger, M.B. Effects of Short-Term Mild Hypercapnia during Head-down Tilt on Intracranial Pressure and Ocular Structures in Healthy Human Subjects. Physiol. Rep. 2017, 5, e13302. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Tepelus, T.; Stenger, M.B.; Lee, S.M.C.; Laurie, S.S.; Liu, J.H.K.; Feiveson, A.H.; Sadda, S.R.; Huang, A.S.; Macias, B.R. Thigh Cuffs as a Countermeasure for Ocular Changes in Simulated Weightlessness. Ophthalmology 2018, 125, 459–460. [Google Scholar] [CrossRef]
- Macias, B.R.; Liu, J.H.K.; Grande-Gutierrez, N.; Hargens, A.R. Intraocular and Intracranial Pressures during Head-down Tilt with Lower Body Negative Pressure. Aviat. Space Environ. Med. 2015, 86, 3–7. [Google Scholar] [CrossRef]
- Liu, J.H.K.; Bouligny, R.P.; Kripke, D.F.; Weinreb, R.N. Nocturnal Elevation of Intraocular Pressure Is Detectable in the Sitting Position. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4439–4442. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Cook, J.; Friberg, T.R. Effect of Inverted Body Position on Intraocular Pressure. Am. J. Ophthalmol. 1984, 98, 784–787. [Google Scholar] [CrossRef]
- Arora, N.; McLaren, J.W.; Hodge, D.O.; Sit, A.J. Effect of Body Position on Epsicleral Venous Pressure in Healthy Subjects. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5151–5156. [Google Scholar] [CrossRef]
- Linder, B.J.; Trick, G.L.; Wolf, M.L. Altering Body Position Affects Intraocular Pressure and Visual Function. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1492–1497. [Google Scholar]
- Greenwald, S.H.; Macias, B.R.; Lee, S.M.C.; Marshall-Goebel, K.; Ebert, D.J.; Liu, J.H.K.; Ploutz-Snyder, R.J.; Alferova, I.V.; Dulchavsky, S.A.; Hargens, A.R.; et al. Intraocular Pressure and Choroidal Thickness Respond Differently to Lower Body Negative Pressure during Spaceflight. J. Appl. Physiol. 2021, 131, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Elwy, R.; Soliman, M.A.; Hasanain, A.A.; Ezzat, A.A.; Elbaroody, M.; Alsawy, M.F.; El Refaee, E. Visual Changes after Space Flight: Is It Really Caused by Increased Intracranial Tension? A Systematic Review. J. Neurosurg. Sci. 2020, 64, 468–479. Available online: https://www.minervamedica.it/en/journals/neurosurgical-sciences/article.php?cod=R38Y2020N05A0468 (accessed on 7 July 2023). [CrossRef] [PubMed]
- Trivli, A.; Koliarakis, I.; Terzidou, C.; Goulielmos, G.N.; Siganos, C.S.; Spandidos, D.A.; Dalianis, G.; Detorakis, E.T. Normal-tension Glaucoma: Pathogenesis and Genetics (Review). Exp. Ther. Med. 2019, 17, 563–574. [Google Scholar] [CrossRef]
- Abe, R.Y.; Gracitelli, C.P.B.; Diniz-Filho, A.; Tatham, A.J.; Medeiros, F.A. Lamina Cribrosa in Glaucoma: Diagnosis and Monitoring. Curr. Ophthalmol. Rep. 2015, 3, 74–84. [Google Scholar] [CrossRef]
- Kim, Y.W.; Jeoung, J.W.; Kim, Y.K.; Park, K.H. Clinical Implications of In Vivo Lamina Cribrosa Imaging in Glaucoma. J. Glaucoma 2017, 26, 753–761. [Google Scholar] [CrossRef]
- Hou, R.; Zhang, Z.; Yang, D.; Wang, H.; Chen, W.; Li, Z.; Sang, J.; Liu, S.; Cao, Y.; Xie, X.; et al. Pressure Balance and Imbalance in the Optic Nerve Chamber: The Beijing Intracranial and Intraocular Pressure (iCOP) Study. Sci. China Life Sci. 2016, 59, 495–503. [Google Scholar] [CrossRef]
- Price, D.A.; Harris, A.; Siesky, B.; Mathew, S. The Influence of Translaminar Pressure Gradient and Intracranial Pressure in Glaucoma: A Review. J. Glaucoma 2020, 29, 141–146. [Google Scholar] [CrossRef]
- Baneke, A.J.; Aubry, J.; Viswanathan, A.C.; Plant, G.T. The Role of Intracranial Pressure in Glaucoma and Therapeutic Implications. Eye 2020, 34, 178–191. [Google Scholar] [CrossRef]
- Chen, W.; Hu, T.; Xu, Q.; Chen, Z.; Zhang, H.; Wang, J. Acute Effects of Intraocular Pressure-Induced Changes in Schlemm’s Canal Morphology on Outflow Facility in Healthy Human Eyes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 36. [Google Scholar] [CrossRef]
- Otto, C. The Visual Impairment Intracranial Pressure (VIIP) Risk in Spaceflight. In Handbook of Bioastronautics; Young, L.R., Sutton, J.P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 641–673. ISBN 978-3-319-12191-8. [Google Scholar]
- Cook, J.A.; Botello, A.P.; Elders, A.; Fathi Ali, A.; Azuara-Blanco, A.; Fraser, C.; McCormack, K.; Margaret Burr, J. Systematic Review of the Agreement of Tonometers with Goldmann Applanation Tonometry. Ophthalmology 2012, 119, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.B. The Non-Contact Tonometer. Its Value and Limitations. Surv. Ophthalmol. 1980, 24, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ethier, C.R.; Yoo, P.; Berdahl, J.P. The Effects of Negative Periocular Pressure on Intraocular Pressure. Exp. Eye Res. 2020, 191, 107928. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Lee, A.G.; Moss, H.E. Head-Down Tilt Bed Rest Studies as a Terrestrial Analog for Spaceflight Associated Neuro-Ocular Syndrome. Front. Neurol. 2021, 12, 648958. [Google Scholar] [CrossRef]
- Ong, J.; Tarver, W.; Brunstetter, T.; Mader, T.H.; Gibson, C.R.; Mason, S.S.; Lee, A. Spaceflight Associated Neuro-Ocular Syndrome: Proposed Pathogenesis, Terrestrial Analogues, and Emerging Countermeasures. Br. J. Ophthalmol. 2023, 107, 895–900. [Google Scholar] [CrossRef]
- Scott, J.M.; Tucker, W.J.; Martin, D.; Crowell, J.B.; Goetchius, E.; Ozgur, O.; Hamilton, S.; Otto, C.; Gonzales, R.; Ritter, M.; et al. Association of Exercise and Swimming Goggles with Modulation of Cerebro-Ocular Hemodynamics and Pressures in a Model of Spaceflight-Associated Neuro-Ocular Syndrome. JAMA Ophthalmol. 2019, 137, 652. [Google Scholar] [CrossRef]
- Omodaka, K.; Takahashi, S.; Matsumoto, A.; Maekawa, S.; Kikawa, T.; Himori, N.; Takahashi, H.; Maruyama, K.; Kunikata, H.; Akiba, M.; et al. Clinical Factors Associated with Lamina Cribrosa Thickness in Patients with Glaucoma, as Measured with Swept Source Optical Coherence Tomography. PLoS ONE 2016, 11, e0153707. [Google Scholar] [CrossRef]
- Paula, A.P.B.; Paula, J.S.; Silva, M.J.L.; Rocha, E.M.; De Moraes, C.G.; Rodrigues, M.L.V. Effects of Swimming Goggles Wearing on Intraocular Pressure, Ocular Perfusion Pressure, and Ocular Pulse Amplitude. J. Glaucoma 2016, 25, 860. [Google Scholar] [CrossRef]
- Ma, K.T.; Chung, W.S.; Seo, K.Y.; Seong, G.J.; Kim, C.Y. The Effect of Swimming Goggles on Intraocular Pressure and Blood Flow within the Optic Nerve Head. Yonsei Med. J. 2007, 48, 807–809. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Nie, Y.; Li, W. Short-Term Effects of Two Types of Goggles on Intraocular Pressure and Anterior Eye Segment Biometrics. BMC Ophthalmol. 2022, 22, 73. [Google Scholar] [CrossRef]
- Morgan, W.H.; Cunneen, T.S.; Balaratnasingam, C.; Yu, D.-Y. Wearing Swimming Goggles Can Elevate Intraocular Pressure. Br. J. Ophthalmol. 2008, 92, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, T.W.; Ferguson, T.J.; Radcliffe, N.M.; Lewis, R.; Schweitzer, J.; Swan, R.; Berdahl, J.P. 8 Hrs Safety Evaluation of A Multi-Pressure Dial in Eyes with Glaucoma: Prospective, Open-Label, Randomized Study. Clin. Ophthalmol. 2019, 13, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Fazio, M.A.; Bianco, G.; Karuppanan, U.; Hubbard, M.; Bruno, L.; Girkin, C.A. The Effect of Negative Pressure on IOP in the Living Human Eye. medRxiv 2022. [Google Scholar] [CrossRef]
- Lee, A.G.; Mader, T.H.; Gibson, C.R.; Tarver, W.; Rabiei, P.; Riascos, R.F.; Galdamez, L.A.; Brunstetter, T. Spaceflight Associated Neuro-Ocular Syndrome (SANS) and the Neuro-Ophthalmologic Effects of Microgravity: A Review and an Update. npj Microgravity 2020, 6, 7. [Google Scholar] [CrossRef]
- Stern, C.; Yücel, Y.H.; zu Eulenburg, P.; Pavy-Le Traon, A.; Petersen, L.G. Eye-Brain Axis in Microgravity and Its Implications for Spaceflight Associated Neuro-Ocular Syndrome. npj Microgravity 2023, 9, 56. [Google Scholar] [CrossRef]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- McMonnies, C.W. The Interaction between Intracranial Pressure, Intraocular Pressure and Lamina Cribrosal Compression in Glaucoma. Clin. Exp. Optom. 2016, 99, 219–226. [Google Scholar] [CrossRef]
- Bakke, E.F.; Hisdal, J.; Semb, S.O. Intraocular Pressure Increases in Parallel with Systemic Blood Pressure during Isometric Exercise. Investig. Ophthalmol. Vis. Sci. 2009, 50, 760–764. [Google Scholar] [CrossRef]
- Natsis, K.; Asouhidou, I.; Nousios, G.; Chatzibalis, T.; Vlasis, K.; Karabatakis, V. Aerobic Exercise and Intraocular Pressure in Normotensive and Glaucoma Patients. BMC Ophthalmol. 2009, 9, 6. [Google Scholar] [CrossRef]
- Chromiak, J.A.; Abadie, B.R.; Braswell, R.A.; Koh, Y.S.; Chilek, D.R. Resistance Training Exercises Acutely Reduce Intraocular Pressure in Physically Active Men and Women. J. Strength Cond. Res. 2003, 17, 715. [Google Scholar]
- Marshall-Bowman, K.; Barratt, M.R.; Gibson, C.R. Ophthalmic Changes and Increased Intracranial Pressure Associated with Long Duration Spaceflight: An Emerging Understanding. Acta Astronaut. 2013, 87, 77–87. [Google Scholar] [CrossRef]
- Anderson, A.P.; Swan, J.G.; Phillips, S.D.; Knaus, D.A.; Kattamis, N.T.; Toutain-Kidd, C.M.; Zegans, M.E.; Fellows, A.M.; Buckey, J.C. Acute Effects of Changes to the Gravitational Vector on the Eye. J. Appl. Physiol. 2016, 120, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; De Dios, Y.; Kofman, I.; Mulavara, A.P.; Bloomberg, J.J.; Seidler, R.D. Head Down Tilt Bed Rest Plus Elevated CO2 as a Spaceflight Analog: Effects on Cognitive and Sensorimotor Performance. Front. Hum. Neurosci. 2019, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Watenpaugh, D.E. Analogs of Microgravity: Head-down Tilt and Water Immersion. J. Appl. Physiol. 2016, 120, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Taibbi, G.; Young, M.; Vyas, R.J.; Murray, M.C.; Lim, S.; Predovic, M.; Jacobs, N.M.; Askin, K.N.; Mason, S.S.; Zanello, S.B.; et al. Opposite Response of Blood Vessels in the Retina to 6° Head-down Tilt and Long-Duration Microgravity. npj Microgravity 2021, 7, 38. [Google Scholar] [CrossRef]
- Lee, A.G.; Mader, T.H.; Gibson, C.R.; Brunstetter, T.J.; Tarver, W.J. Space Flight-Associated Neuro-Ocular Syndrome (SANS). Eye 2018, 32, 1164–1167. [Google Scholar] [CrossRef]
- Ong, J.; Tavakkoli, A.; Zaman, N.; Kamran, S.A.; Waisberg, E.; Gautam, N.; Lee, A.G. Terrestrial Health Applications of Visual Assessment Technology and Machine Learning in Spaceflight Associated Neuro-Ocular Syndrome. npj Microgravity 2022, 8, 37. [Google Scholar] [CrossRef]
- Ong, J.; Zaman, N.; Waisberg, E.; Kamran, S.A.; Lee, A.G.; Tavakkoli, A. Head-Mounted Digital Metamorphopsia Suppression as a Countermeasure for Macular-Related Visual Distortions for Prolonged Spaceflight Missions and Terrestrial Health. Wearable Technol. 2022, 3, e26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masalkhi, M.; Ong, J.; Waisberg, E.; Berdahl, J.; Lee, A.G. Intraocular Pressure during Spaceflight and Risk of Glaucomatous Damage in Prolonged Microgravity. Encyclopedia 2023, 3, 1187-1196. https://doi.org/10.3390/encyclopedia3040086
Masalkhi M, Ong J, Waisberg E, Berdahl J, Lee AG. Intraocular Pressure during Spaceflight and Risk of Glaucomatous Damage in Prolonged Microgravity. Encyclopedia. 2023; 3(4):1187-1196. https://doi.org/10.3390/encyclopedia3040086
Chicago/Turabian StyleMasalkhi, Mouayad, Joshua Ong, Ethan Waisberg, John Berdahl, and Andrew G. Lee. 2023. "Intraocular Pressure during Spaceflight and Risk of Glaucomatous Damage in Prolonged Microgravity" Encyclopedia 3, no. 4: 1187-1196. https://doi.org/10.3390/encyclopedia3040086
APA StyleMasalkhi, M., Ong, J., Waisberg, E., Berdahl, J., & Lee, A. G. (2023). Intraocular Pressure during Spaceflight and Risk of Glaucomatous Damage in Prolonged Microgravity. Encyclopedia, 3(4), 1187-1196. https://doi.org/10.3390/encyclopedia3040086