Exploring Potentials for Bioresource and Bioenergy Recovery from Vinasse, the “New” Protagonist in Brazilian Sugarcane Biorefineries
Abstract
:1. Residual Stream Management in Sugarcane Biorefineries
2. Sugarcane Vinasse: Biodegradable Organic Fraction and Nutrients
3. (Bio)Technological Applications (Other Than Biodigestion) for Sugarcane Vinasse
4. Biodigestion as the Core Technology for Processing Sugarcane Vinasse
4.1. Fundamentals of Anaerobic Processes

| Microbial Group | Reaction | ΔG°′ (kJ mol−1) | Equation |
|---|---|---|---|
| Fermentative bacteria | Glucose + 2H2O → 2Acetate + 4H2 + 2CO2 | −206.0 | (1) 1 |
| Glucose → Butyrate + 2H2 + 2CO2 | −254.0 | (2) 1 | |
| Glucose + 2H2 → 2Propionate + 2H2O | −279.4 | (3) 1 | |
| Glucose → 2Lactate + 2H+ | −198.0 | (4) 2 | |
| Glucose → 2Ethanol + 2CO2 | −164.8 | (5) 3 | |
| Glucose + H2O → Ethanol + Acetate + 2H2 + 2CO2 | −205.2 | (6) 3 | |
| Acetate + 2Lactate → H2 + 1.5Butyrate + 2CO2 + H2O | −156.6 | (7) 4 | |
| 3Lactate → 2Propionate + Acetate + CO2 + H2O | −170.0 | (8) 5 | |
| Acetogenic bacteria | Propionate + 2H2O → Acetate + 3H2 + CO2 | +76.2 | (9) 3 |
| Butyrate + 2H2O → 2Acetate + 2H2 | +48.4 | (10) 3 | |
| Ethanol + H2O → Acetate + 2H2 | +9.6 | (11) 3 | |
| Lactate + 2H2O → Acetate + 3HCO3− + 2H2 | −4.2 | (12) 3 | |
| Glycerol + 2H2O → Acetate + HCO3− + 2H+ + 2H2 | −73.2 | (13) 6 | |
| Homoacetogenic bacteria | 4H2 + 2CO2 → Acetate + 2H2O | −104.0 | (14) 3 |
| Acetoclastic methanogens | Acetate → CH4 + H2O | −31.0 | (15) 3 |
| Hydrogenotrophic methanogens | 4H2 + CO2 → CH4 + 2H2O | −135.0 | (16) 3 |
| Sulfate-reducing bacteria | SO42− + Acetate → HS− + 2HCO3− | −47.3 | (17) 7 |
| SO42− + 2Butyrate → HS− + H+ + 4Acetate | −55.5 | (18) 7 | |
| SO42− + 2Ethanol → HS− + 2H2O + H+ + 2Acetate | −132.7 | (19) 7 | |
| 3SO42− + 4Propionate → 3HS− + 4HCO3− + H+ + 4Acetate | −150.6 | (20) 7 | |
| SO42− + 4H2 + H+ → HS− + 4H2O | −152.2 | (21) 7 | |
| SO42− + 2Lactate → HS− + 2HCO3− + H+ + 2Acetate | −160.1 | (22) 7 | |
| 3SO42− + 2Ethanol → 3HS− + 4HCO3− + H+ + 2H2O | −227.3 | (23) 7 | |
| Glycerol + 1.25SO42− → 0.5Acetate + 1.5H2CO3 +0.5HCO3− + 1.25HS− + 0.75OH− + H2O | −424.5 | (24) 8 |
4.2. Biodigestion of Vinasse in Brazil: Background and Current State of the Research
4.3. Predicting the Energetic Potential of Sugarcane Vinasse
4.3.1. Economics of Vinasse-Derived Bioenergy
4.4. Phase Separation: Enhancing Resource Recovery from Vinasse
4.5. Alternative Pathways for Bioresource Recovery from Vinasse via Acidogenesis
4.6. Additional Opportunities for Sugarcane Vinasse Exploitation via Biodigestion
5. Outlook: Implementing Biodigestion in Sugarcane Biorefineries
- Ethanol plants are capital intensive and require several unit processes (e.g., fermentation, centrifugation, and distillation) that demand high levels of energy, while biodigestion requires much less investment and energy inputs [46,269]. Fuess et al. [50] estimated that the installation costs of biodigestion systems coupled with power plants for recovering bioenergy from vinasse would be less than 10% of the total investment with large scale sugarcane biorefineries (with milling capacities of 4 MTC and 1.7 MTC in the harvesting and inter-harvesting periods, respectively). This particular analysis already considers the installation of two-phase anaerobic plants. Similarly, Junqueira et al. [267] also indicated that the biodigestion of vinasse coupled with the recovery of bioenergy accounts for a minimal fraction of the total investment in sugarcane biorefineries, and ranges from 3.4–7.5% depending on the technological package considered for exploiting the biogas–CH4 (cogeneration of electricity and steam in boilers, electricity generation in engines, bioCH4 production for diesel replacement and bioCH4 production, and further injection into the gas grid).
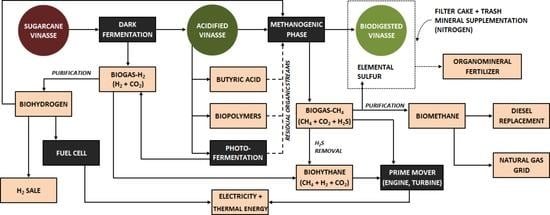
- The energy potential of 1 ton of cleaned sugarcane is approximately 1718 MCal, which is characterized by the fractions of sugars (153 kg, 608 Mcal), bagasse with 50% moisture (216 kg, 598 Mcal), and trash with 15% moisture (165 kg, 512 Mcal) [282]. Thus, the maximum theoretical energy recoverable from sugarcane through 1G ethanol production could reach roughly 35%, when assuming the conversion of the total fraction of sugars and without taking into consideration energy losses due to parallel metabolic pathways and yeast growth. However, in practical aspects, the ethanol productivity (in which all “metabolic losses” are included) currently obtained in 1G sugarcane biorefineries (82 L TC−1 [5]) leads to an effective energy recovery below 25% of the total energy in sugarcane (416 Mcal) when considering a LHV of 21.22 MJ L−1 for ethanol [62]. Conversely, van Haandel and Catunda [46] and Wilkie [269] indicated that biodigestion may lead to a more efficient use of the sugars present in sugarcane. The direct conversion of the juice into biogas–CH4 would recover approximately 50% of the total energy present in the sugarcane. Fuess et al. [281] associated an energy return on investment (EROI) ratio to the direct biogas production (and further conversion into electricity) from juice and juice/bagasse as four times higher than the one observed in ethanol production. In practical terms, the results indicate that producing energy from biogas is more efficient than using ethanol, which fully suits one of the UN Sustainable Development Goals (#7: Ensure access to affordable, reliable, sustainable, and modern energy for all [283]). The further conversion of bagasse into biogas in anaerobic systems, either via solid-state biodigestion [284,285] or via sugar-rich hydrolisates [10,11,12,286] (Figure 5), could enhance the energy recovery to 60–80% [46,269], while the integration of 1G and 2G sugarcane biorefineries may increase the energy recovery through ethanol production to only 30–34% (i.e., 518–589 Mcal, considering global ethanol productivities in the range of 102–116 L TC−1 [5]). Some energy recovery improvements in 2G ethanol plants could be achieved through the biodigestion of 2G vinasse and pentose liquor (HSW streams depicted in Figure 1b), as proposed elsewhere [66].
- The better performance of biodigestion compared to yeast-driven fermentation in terms of energy extraction directly affects the energy potential of the produced biofuels, because the LHV of CH4 (50.00 MJ kg−1 [223]) is approximately double the value observed for ethanol (26.90 MJ kg−1 [223]). Moreover, biogas applications (either purified or non-purified) are diverse (Figure 5), insofar as the layout of the biorefineries could be designed according to the most profitable option, i.e., the production of biochemicals (through acidogenesis), electricity generation from biogas–CH4, and bioCH4 production. In practical terms, biogas-based biorefineries are much more flexible than those producing ethanol as the main biofuel [270,281], which fully suits the biorefining concept presented elsewhere [41]: “the sustainable processing of biomass into a spectrum of marketable products, which means: energy, materials, chemicals, food and feed”. Naturally, the implementation of sugarcane biorefineries using biodigestion as the core process does not exclude industrial plants based on the traditional production of sugar and/or ethanol, in which the biodigestion of vinasse should necessarily be incorporated. In short, the aforementioned aspects could be carefully considered in future greenfield projects of the sugar and ethanol sector to consider biodigestion as an efficient step for exploiting sugarcane, either directly or from the residual fractions.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lima, M.A.P.; Bonomi, A.; Cavalett, O.; Cunha, M.P. Background. In Virtual Biorefinery: An Optimization Strategy for Renewable Carbon Valorization; Bonomi, A., Cavalett, O., Cunha, M.P., Lima, M.A.P., Eds.; Springer: London, UK, 2016; pp. 1–4. [Google Scholar]
- Dias, M.O.S.; Modesto, M.; Ensinas, A.V.; Nebra, S.A.; Maciel Filho, R.; Rossell, C.E.V. Improving bioethanol production from sugarcane: Evaluation of distillation, thermal integration and cogeneration systems. Energy 2011, 36, 3691–3703. [Google Scholar] [CrossRef]
- Moraes, B.S.; Zaiat, M.; Bonomi, A. Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: Challenges and perspectives. Renew. Sustain. Energy Rev. 2015, 44, 888–903. [Google Scholar] [CrossRef]
- Junqueira, T.L.; Cavalett, O.; Bonomi, A. The virtual sugarcane biorefinery—A simulation tool to support public policies formulation in bioenergy. Ind. Biotechnol. 2016, 12, 62–67. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Junqueira, T.L.; Cavalett, O.; Cunha, M.P.; Jesus, C.D.F.; Rossell, C.E.V.; Maciel Filho, R.; Bonomi, A. Integrated versus stand-alone second generation ethanol production from sugarcane bagasse and trash. Bioresour. Technol. 2012, 103, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, M.O.S.; Junqueira, T.L.; Cavalett, O.; Pavanello, L.G.; Cunha, M.P.; Jesus, C.D.F.; Maciel Filho, R.; Bonomi, A. Biorefineries for the production of first and second generation ethanol and electricity from sugarcane. Appl. Energy 2013, 109, 72–78. [Google Scholar] [CrossRef]
- Morais, E.R.; Junqueira, T.L.; Sampaio, I.L.M.; Dias, M.O.S.; Rezende, M.C.A.F.; Jesus, C.D.F.; Klein, B.C.; Gómez, E.O.; Mantelatto, P.E.; Maciel Filho, R.; et al. Biorefinery alternatives. In Virtual Biorefinery: An Optimization Strategy for Renewable Carbon Valorization; Bonomi, A., Cavalett, O., Cunha, M.P., Lima, M.A.P., Eds.; Springer: London, UK, 2016; pp. 53–132. [Google Scholar]
- Volpi, M.P.C.; Ferraz, A.D.N., Jr.; Franco, T.T.; Moraes, B.S. Operational and biochemical aspects of co-digestion (co-AD) from sugarcane vinasse, filter cake, and deacetylation liquor. Appl. Microbiol. Biotechnol. 2021, 105, 8969–8987. [Google Scholar] [CrossRef]
- Lora, E.E.S.; Andrade, R.V.; Ángel, J.D.M.; Leite, M.A.H.; Rocha, M.H.; Sales, C.A.V.B.; Mendoza, M.A.G.; Coral, D.S.O. Gasification and pyrolysis for converting biomass into electricity and biofuels. In Biofuels; Lora, E.E.S., Venturini, O.J., Eds.; Interciência: Rio de Janeiro, Brazil, 2012; Volume 1, pp. 411–498. [Google Scholar]
- Baêta, B.E.L.; Lima, D.R.S.; Balena Filho, J.G.; Adarme, O.F.H.; Gurgel, L.V.A.; Aquino, S.F. Evaluation of hydrogen and methane production from sugarcane bagasse hemicellulose hydrolysates by two-stage anaerobic digestion process. Bioresour. Technol. 2016, 218, 436–446. [Google Scholar] [CrossRef]
- Bolado-Rodríguez, S.; Toquero, C.; Martín-Juárez, J.; Travaini, R.; García-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef]
- Jafari, O.; Zilouei, H. Enhanced biohydrogen and subsequent biomethane production from sugarcane bagasse using nano-titanium dioxide pretreatment. Bioresour. Technol. 2016, 214, 670–678. [Google Scholar] [CrossRef]
- Janke, L.; Weinrich, S.; Leite, A.F.; Sträuber, H.; Radetski, C.M.; Nikolausz, M.; Nelles, M.; Stinner, W. Year-round biogas production in sugarcane biorefineries: Process stability, optimization and performance of a two-stage reactor system. Energy Convers. Manag. 2018, 168, 188–199. [Google Scholar] [CrossRef]
- Almeida, A.B., Jr.; Nascimento, C.W.A.; Sobral, M.F.; Silva, F.B.V.; Gomes, W.A. Soil fertility and uptake of nutrients by sugarcane fertilized with filter cake. Rev. Bras. Eng. Agríc. Ambient. 2011, 15, 1004–1013. (In Portuguese) [Google Scholar]
- Contreras, A.M.; Rosa, E.; Pérez, M.; van Langenhove, H.; Dewulf, J. Comparative life cycle assessment of four alternatives for using by-products of cane sugar production. J. Clean. Prod. 2009, 17, 772–779. [Google Scholar] [CrossRef]
- España-Gamboa, E.; Mijangos-Cortes, J.; Barahona-Perez, L.; Dominguez-Maldonado, J.; Hernández-Zarate, G.; Alzate-Gaviria, L. Vinasses: Characterization and treatments. Waste Manag. Res. 2011, 29, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; Garcia, M.L. Implications of stillage land disposal: A critical review on the impacts of fertigation. J. Environ. Manag. 2014, 145, 210–229. [Google Scholar] [CrossRef]
- Fuess, L.T.; Altoé, M.E.; Felipe, M.C.; Garcia, M.L. Pros and cons of fertirrigation with in natura sugarcane vinasse: Do improvements in soil fertility offset environmental and bioenergy losses? J. Clean. Prod. 2021, 319, 128684. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Maciel Filho, R.; Mantelatto, P.E.; Cavalett, O.; Rossell, C.E.V.; Bonomi, A.; Leal, M.R.L.V. Sugarcane processing for ethanol and sugar in Brazil. Environ. Dev. 2015, 15, 35–51. [Google Scholar] [CrossRef]
- Oliveira, B.G.; Carvalho, J.L.N.; Cerri, C.E.P.; Cerri, C.C.; Feigl, B.J. Soil greenhouse gas fluxes from vinasse application in Brazilian sugarcane areas. Geoderma 2013, 200–201, 77–84. [Google Scholar] [CrossRef]
- Carvalho, J.L.; Oliveira, B.G.; Cantarella, H.; Chagas, M.F.; Gonzaga, L.C.; Lourenço, K.S.; Bordonal, R.O.; Bonomi, A. Implications of regional N2O-N emission factors on sugarcane ethanol emissions and granted decarbonization certificates. Renew. Sustain. Energy Rev. 2021, 149, 111423. [Google Scholar] [CrossRef]
- Oliveira, B.G.; Lourenço, K.S.; Carvalho, J.L.N.; Gonzaga, L.C.; Teixeira, M.C.; Tamara, A.F.; Cantarella, H. Soil pH does not interfere with nitrification inhibitor efficiency for reducing N2O emissions from soils treated with concentrated vinasse and urea. Geoderma 2022, 426, 116087. [Google Scholar] [CrossRef]
- Wilkie, A.C.; Riedesel, K.J.; Owens, J.M. Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biomass Bioenergy 2000, 19, 63–102. [Google Scholar] [CrossRef]
- Rodrigues, I.J.; Fuess, L.T.; Biondo, L.; Santesso, C.A.; Garcia, M.L. Coagulation-flocculation of anaerobically treated sugarcane stillage. Desalin. Water Treat. 2014, 52, 4111–4121. [Google Scholar] [CrossRef]
- Guerreiro, L.F.; Rodrigues, C.S.D.; Duda, R.M.; Oliveira, R.A.; Boaventura, R.A.R.; Madeira, L.M. Treatment of sugarcane vinasse by combination of coagulation/flocculation and Fenton’s oxidation. J. Environ. Manag. 2016, 181, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayas, T.; Romero, V.; Salgado, L.; Meraz, M.; Morales, U. Applicability of coagulation/flocculation and electrochemical processes to the purification of biologically treated vinasse effluent. Sep. Purif. Technol. 2007, 57, 270–276. [Google Scholar] [CrossRef]
- Seixas, F.L.; Gimenes, M.L.; Fernades-Machado, N.R.C. Treatment of vinasse by adsorption on carbon from sugar cane bagasse. Quim. Nova 2016, 39, 172–179. (In Portuguese) [Google Scholar] [CrossRef]
- Aquino, S.; Pires, E.C. Assessment of ozone as a pretreatment to improve anaerobic digestion of vinasse. Braz. J. Chem. Eng. 2016, 33, 279–285. [Google Scholar] [CrossRef]
- Asaithambi, P.; Susree, M.; Saravanathamizhan, R.; Matheswaran, M. Ozone assisted electrocoagulation for the treatment of distillery effluent. Desalination 2012, 297, 1–7. [Google Scholar] [CrossRef]
- Yavuz, Y. EC and EF processes for the treatment of alcohol distillery wastewater. Sep. Purif. Technol. 2007, 53, 135–140. [Google Scholar] [CrossRef]
- de Bazúa, C.D.; Cabrero, M.A.; Poggi, H.M. Vinasses biological treatment by anaerobic and aerobic processes: Laboratory and pilot-plant tests. Bioresour. Technol. 1991, 35, 87–93. [Google Scholar] [CrossRef]
- Ferreira, L.F.R.; Aguiar, M.M.; Messias, T.G.; Pompeu, G.B.; Lopez, A.M.Q.; Silva, D.P.; Monteiro, R.T. Evaluation of sugar-cane vinasse treated with Pleurotus sajor-caju utilizing aquatic organisms as toxicological indicators. Ecotox. Environ. Saf. 2011, 74, 132–137. [Google Scholar] [CrossRef]
- Aquino, S.; Fuess, L.T.; Pires, E.C. Media arrangement impacts cell growth in anaerobic fixed-bed reactors treating sugarcane vinasse: Structured vs. randomic biomass immobilization. Bioresour. Technol. 2017, 235, 219–228. [Google Scholar] [CrossRef]
- Craveiro, A.M.; Soares, H.M.; Schmidell, W. Technical aspects and cost estimations for anaerobic systems treating vinasse and brewery/soft drink wastewaters. Water Sci. Technol. 1986, 18, 123–134. [Google Scholar] [CrossRef]
- Ferraz, A.D.N., Jr.; Koyama, M.H.; Araújo, M.M., Jr.; Zaiat, M. Thermophilic anaerobic digestion of raw sugarcane vinasse. Renew. Energy 2016, 89, 245–252. [Google Scholar]
- Fuess, L.T.; Kiyuna, L.S.M.; Ferraz, A.D.N., Jr.; Persinoti, G.F.; Squina, F.M.; Garcia, M.L.; Zaiat, M. Thermophilic two-phase anaerobic digestion using innovative fixed-bed reactor for enhanced organic matter removal and bioenergy recovery from sugarcane vinasse. Appl. Energy 2017, 189, 480–491. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.E.; Fuzaro, G.; Polegato, A.R. Thermophilic anaerobic digestion of vinasse in pilot plant UASB reactor. Water Sci. Technol. 1992, 25, 213–222. [Google Scholar] [CrossRef]
- Del Nery, V.; Alves, I.; Damianovic, M.H.R.Z.; Pires, E.C. Hydraulic and organic rates applied to pilot scale UASB reactor for sugar cane vinasse degradation and biogas generation. Biomass Bioenergy 2018, 119, 411–417. [Google Scholar] [CrossRef]
- Ramos, L.R.; Lovato, G.; Rodrigues, J.A.D.; Silva, E.L. Anaerobic digestion of vinasse in fluidized bed reactors: Process robustness between two-stage thermophilic-thermophilic and thermophilic-mesophillic systems. J. Clean. Prod. 2021, 314, 128066. [Google Scholar] [CrossRef]
- Barbosa, M.Y.U.; Alves, I.; Del Nery, V.; Sakamoto, I.K.; Pozzi, E.; Damianovic, M.H.R.Z. Methane production in a UASB reactor from sugarcane vinasse: Shutdown or exchanging substrate for molasses during the off-season? J. Water Process Eng. 2022, 47, 102664. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Munoz-Paez, K.M.; Escamilla-Alvarado, C.; Robledo-Narváez, P.N.; Ponce-Noyola, M.T.; Calva-Calva, G.; Ríos-Leal, E.; Galíndez-Mayer, J.; Estrada-Vázquez, C.; Ortega-Clemente, A.; et al. Biohydrogen, biomethane and bioelectricity as crucial components of biorefinery of organic wastes: A review. Waste Manag. Res. 2014, 32, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Choi, H.S.; Park, C.; Kim, S.W. Current states and prospects of organic waste utilization for biorefineries. Renew. Sustain. Energy Rev. 2015, 49, 335–349. [Google Scholar] [CrossRef]
- Sánchez, F.E.; Fuess, L.T.; Cavalcante, G.S.; Adorno, M.A.T.; Zaiat, M. Value-added soluble metabolite production from sugarcane vinasse within the carboxylate platform: An application of the anaerobic biorefinery beyond biogas production. Fuel 2021, 286, 119378. [Google Scholar] [CrossRef]
- Fuess, L.T.; Kiyuna, L.S.M.; Garcia, M.L.; Zaiat, M. Operational strategies for long-term biohydrogen production from sugarcane stillage in continuous acidogenic packed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 8132–8145. [Google Scholar] [CrossRef] [Green Version]
- Siles, J.A.; García-García, I.; Martín, A.; Martín, M.A. Integrated ozonation and biomethanization treatments of vinasse derived from ethanol manufacturing. J. Hazard. Mater. 2011, 188, 247–253. [Google Scholar] [CrossRef] [PubMed]
- van Haandel, A.C.; Catunda, P.F.C. Profitability increase of alcohol distilleries by the rational use of byproducts. Water Sci. Technol. 1994, 29, 117–124. [Google Scholar] [CrossRef]
- Willington, I.P.; Marten, G.G. Options for handling stillage waste from sugar-based fuel ethanol production. Resour. Conserv. 1982, 8, 111–129. [Google Scholar] [CrossRef]
- Fuess, L.T.; Garcia, M.L. Bioenergy from stillage anaerobic digestion to enhance the energy balance ratio of ethanol production. J. Environ. Manag. 2015, 162, 102–114. [Google Scholar] [CrossRef]
- Fuess, L.T.; Zaiat, M. Economics of anaerobic digestion for processing sugarcane vinasse: Applying sensitivity analysis to increase process profitability in diversified biogas applications. Process Saf. Environ. Prot. 2018, 115, 27–37. [Google Scholar] [CrossRef]
- Fuess, L.T.; Klein, B.C.; Chagas, M.F.; Rezende, M.C.A.F.; Garcia, M.L.; Bonomi, A.; Zaiat, M. Diversifying the technological strategies for recovering bioenergy from the two-phase anaerobic digestion of sugarcane vinasse: An integrated techno-economic and environmental approach. Renew. Energy 2018, 122, 674–687. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.R.; Lovato, G.; Rodrigues, J.A.D.; Silva, E.L. Scale-up and energy estimations of single- and two-stage vinasse anaerobic digestion systems for hydrogen and methane production. J. Clean. Prod. 2022, 349, 131459. [Google Scholar] [CrossRef]
- Borges, A.V.; Fuess, L.T.; Alves, I.; Takeda, P.Y.; Damianovic, M.H.R.Z. Co-digesting sugarcane vinasse and distilled glycerol to enhance bioenergy generation in biofuel-producing plants. Energy Convers. Manag. 2021, 250, 114897. [Google Scholar] [CrossRef]
- Takeda, P.Y.; Oliveira, C.A.; Dias, M.E.S.; Paula, C.T.; Borges, A.V.; Damianovic, M.H.R.Z. Enhancing the energetic potential of sugarcane biorefinery exchanging vinasse and glycerol in sugarcane off-season in an anaerobic reactor. Renew. Energy 2022, 195, 1218–1229. [Google Scholar] [CrossRef]
- Aguiar, M.M.; Ferreira, L.F.R.; Monteiro, R.T.R. Use of vinasse and sugarcane bagasse for the production of enzymes by lignocellulolytic fungi. Braz. Arch. Biol. Technol. 2010, 53, 1245–1254. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.G.; Garcia-Cruz, C.H. Properties of a biosurfactant produced by Bacillus pumilus using vinasse and waste frying oil as alternative carbon sources. Braz. Arch. Biol. Technol. 2013, 56, 155–160. [Google Scholar] [CrossRef]
- Nitayavardhana, S.; Khanal, S.K. Innovative biorefinery concept for sugar-based ethanol industries: Production of protein-rich fungal biomass on vinasse as an aquaculture feed ingredient. Bioresour. Technol. 2010, 101, 9078–9085. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N.N.V.; Farenzena, M.; Trierweiler, J.O. Growth of microalgae Scenedesmus sp. in ethanol vinasse. Braz. Arch. Biol. Technol. 2014, 57, 630–635. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, J.C.; Braga, M.Q.; Ázara, M.S.; Garcia, K.J.; Alencar, S.N.M.; Ramos, T.S.; Siniscalchi, L.A.B.; Assemany, P.P.; Ensinas, A.V. Recovery of vinasse with combined microalgae cultivation in a conceptual energy-efficient industrial plant: Analysis of related process considerations. Renew. Sustain. Energy Rev. 2022, 155, 111904. [Google Scholar] [CrossRef]
- Döll, M.M.R.; Foresti, E. Effect of the sodium bicarbonate in the treatment of vinasse in AnSBBR operated at 55 and 35 °C. Eng. Sanit. Ambient. 2010, 15, 275–282. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Siqueira, L.M.; Damiano, E.S.G.; Silva, E.L. Influence of organic loading rate on the anaerobic treatment of sugarcane vinasse and biogas production in fluidized bed reactor. J. Environ. Sci. Health A 2013, 48, 1707–1716. [Google Scholar] [CrossRef]
- Albanez, R.; Chiaranda, B.C.; Ferreira, R.G.; França, A.L.P.; Honório, C.D.; Rodrigues, J.A.D.; Ratusznei, S.M.; Zaiat, M. Anaerobic biological treatment of vinasse for environmental compliance and methane production. Appl. Biochem. Biotechnol. 2016, 178, 21–43. [Google Scholar] [CrossRef]
- Fuess, L.T.; Garcia, M.L. Anaerobic digestion of stillage to produce bioenergy in the sugarcane-to-ethanol industry. Environ. Technol. 2014, 35, 333–339. [Google Scholar] [CrossRef]
- Moraes, B.S.; Junqueira, T.L.; Pavanello, L.G.; Cavalett, O.; Mantelatto, P.E.; Bonomi, A.; Zaiat, M. Anaerobic digestion of vinasse from sugarcane biorefineries in Brazil from energy, environmental, and economic perspectives: Profit or expense? Appl. Energy 2014, 113, 825–835. [Google Scholar] [CrossRef]
- Salomon, K.R.; Lora, E.E.S.; Rocha, M.H.; del Olmo, O.A. Cost calculations for biogas from vinasse biodigestion and its energy utilization. Sugar Ind. 2011, 136, 217–223. [Google Scholar] [CrossRef]
- van Haandel, A.C. Integrated energy production and reduction of the environmental impact at alcohol distillery plants. Water Sci. Technol. 2005, 52, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Longati, A.A.; Lino, A.R.A.; Giordano, R.C.; Furlan, F.F.; Cruz, A.J.G. Biogas production from anaerobic digestion of vinasse in sugarcane biorefinery: A techno-economic and environmental analysis. Waste Biomass Valor. 2020, 11, 4573–4591. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Oliveira, G.H.D.; Zaiat, M.; Rodrigues, J.A.D.; Ramsay, J.A.; Ramsay, B.A. Towards the production of mcl-PHA with enriched dominant monomer content: Process development for the sugarcane biorefinery context. J. Polym. Environ. 2020, 28, 844–853. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Hoff, D.N. Closing the matter and energy cycle in the sugar/alcohol sector. Sustain. Debate 2013, 4, 215–236. (In Portuguese) [Google Scholar] [CrossRef]
- Christofoletti, C.A.; Escher, J.P.; Correia, J.E.; Marinho, J.F.U.; Fontanetti, C.S. Sugarcane vinasse: Environmental implications of its use. Waste Manag. 2013, 33, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.J.; Greenfield, P.F. Utilisation, treatment and disposal of distillery wastewater. Water Res. 1980, 14, 257–277. [Google Scholar] [CrossRef]
- Dowd, M.K.; Johansen, S.L.; Cantarella, L.; Reilly, P.J. Low molecular weight organic composition of ethanol stillage from sugarcane molasses, citrus waste, and sweet whey. J. Agric. Food Chem. 1994, 42, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Navarro, A.R.; Sepúlveda, M.C.; Rubio, M.C. Bio-concentration of vinasse from the alcoholic fermentation of sugar cane molasses. Waste Manag. 2000, 20, 581–585. [Google Scholar] [CrossRef]
- Melo, H.F. Response to Acid Stress in Industrial Fermentation Yeasts. Ph.D. Thesis, Federal University of Pernambuco, Recife, Brazil, 2006. (In Portuguese). [Google Scholar]
- Pereira, I.M. Study of Some Parameters for Glycerol Precipitation by Complexation with Zirconium in Vinasses from Fuel Alcohol and Spirits and Weak Leaches of Soap Manufacture. Master’s Thesis, University of Campinas, Campinas, Brazil, 1991. (In Portuguese). [Google Scholar]
- Bonini, M.A. Heterotrophic Cultivation of Aphanothece microscopica Nägeli and Chlorella vulgaris in Different Carbon Sources and Vinasse. Master’s Thesis, Federal University of São Carlos, Araras, Brazil, 2012. (In Portuguese). [Google Scholar]
- Fuess, L.T.; Zaiat, M.; Nascimento, C.A.O. Novel insights on the versatility of biohydrogen production from sugarcane vinasse via thermophilic dark fermentation: Impacts of pH-driven operating strategies on acidogenesis metabolite profiles. Bioresour. Technol. 2019, 286, 121379. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.S.; Zaiat, M.; Nascimento, C.A.O.; Fuess, L.T. Does sugarcane vinasse composition variability affect the bioenergy yield in anaerobic systems? A dual kinetic-energetic assessment. J. Clean. Prod. 2019, 240, 118005. [Google Scholar] [CrossRef]
- Piffer, M.A.; Zaiat, M.; Nascimento, C.A.O.; Fuess, L.T. Dynamics of sulfate reduction in the thermophilic dark fermentation of sugarcane vinasse: A biohydrogen-independent approach targeting enhanced bioenergy production. J. Environ. Chem. Eng. 2021, 9, 105956. [Google Scholar] [CrossRef]
- Borges, A.V.; Fuess, L.T.; Takeda, P.Y.; Alves, I.; Dias, M.E.S.; Damianovic, M.H.R.Z. Co-digestion of biofuel by-products: Enhanced biofilm formation maintains high organic matter removal when methanogenesis fails. J. Environ. Manag. 2022, 310, 114768. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; Piffer, M.A.; Zaiat, M.; Nascimento, C.A.O. Phase separation enhances bioenergy recovery in sugarcane vinasse biodigestion: Absolute or relative truth? Bioresour. Technol. Rep. 2022, 18, 101026. [Google Scholar] [CrossRef]
- Moraes, B.S.; Santos, G.M.; Delforno, T.P.; Fuess, L.T.; Silva, A.J. Enriched microbial consortia for dark fermentation of sugarcane vinasse towards value-added short-chain organic acids and alcohol production. J. Biosci. Boeng. 2019, 127, 594–601. [Google Scholar] [CrossRef]
- Serafim, F.A.T.; Buchviser, S.F.; Galinaro, C.A.; Franco, D.W.; Novaes, F.V. Organic acids in sugarcane spirits’ fractions produced in stills and columns. Quim. Nova 2011, 34, 28–32. (In Portuguese) [Google Scholar] [CrossRef]
- Basso, C.J.; Santi, A.L.; Lamego, F.P.; Somavilla, L.; Brigo, T.J. Vinasse as a source of potassium: Black oat/corn silage/short-season corn succession response and soil chemical alterations in the Northwest region of the state of Rio Grande do Sul. Cienc. Rural 2013, 43, 596–602. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Barth, D.; Monteiro, A.R.S.; Costa, M.M.; Virkajärvi, I.; Sacon, V.; Wilhelmsom, A. DesinFix TM 135 in fermentation process for bioethanol production. Braz. J. Microbiol. 2014, 45, 323–325. [Google Scholar] [CrossRef] [Green Version]
- Ceccato-Antonini, S.R. Conventional and nonconventional strategies for controlling bacterial contamination in fuel ethanol fermentations. World J. Microbiol. Biotechnol. 2018, 34, 80. [Google Scholar] [CrossRef]
- Kiyuna, L.S.M.; Fuess, L.T.; Zaiat, M. Unraveling the influence of the COD/sulfate ratio on organic matter removal and methane production from the biodigestion of sugarcane vinasse. Bioresour. Technol. 2017, 232, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; Rodrigues, I.J.; Garcia, M.L. Fertirrigation with sugarcane vinasse: Foreseeing potential impacts on soil and water resources through vinasse characterization. J. Environ. Sci. Health A 2017, 52, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Brito, F.L.; Rolim, M.M.; Silva, J.A.A.; Pedrosa, E.M.R. Quality of percolate from soils after application of different doses and incubation time of vinasse. Rev. Bras. Eng. Agríc. Ambient. 2007, 11, 318–323. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Brito, F.L.; Rolim, M.M.; Pedrosa, E.M.R. Effect of vinasse application in chemical characteristics of soils from forest zone of Pernambuco. Rev. Bras. Ciênc. Agrár. 2009, 4, 456–462. (In Portuguese) [Google Scholar]
- Camilotti, F.; Marques, M.O.; Andrioli, I.; Silva, A.R.; Tasso, L.C., Jr.; Nobile, F.O. Heavy metals accumulation in sugarcane after application in sewage sludge and vinasse. Eng. Agríc. 2007, 27, 284–293. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Gunkel, G.; Kosmol, J.; Sobral, M.; Rohn, H.; Montenegro, S.; Aureliano, J. Sugar cane industry as a source of water pollution—Case study on the situation in Ipojuca river, Pernambuco, Brazil. Water Air Soil Poll. 2007, 180, 261–269. [Google Scholar] [CrossRef]
- Lyra, M.R.C.C.; Rolim, M.M.; Silva, J.A.A. Toposequence of soils fertigated with stillage: Contribution towards the quality of ground water table. Rev. Bras. Eng. Agríc. Ambient. 2003, 7, 525–532. (In Portuguese) [Google Scholar] [CrossRef]
- Miranda, T.L.; Pedrosa, E.M.R.; Silva, E.F.F.; Rolim, M.M. Physical and biological alterations in sugarcane cultivated soil after harvest and vinasse application. Rev. Bras. Ciênc. Agrár. 2012, 7, 150–158. (In Portuguese) [Google Scholar]
- Moore, C.C.S.; Nogueira, A.R.; Kulay, L. Environmental and energy assessment of the substitution of chemical fertilizers for industrial wastes of ethanol production in sugarcane cultivation in Brazil. Int. J. Life Cycle Assess. 2017, 22, 628–643. [Google Scholar] [CrossRef]
- Ribeiro, B.T.; Lima, J.M.; Guilherme, L.R.G.; Julião, L.G.F. Lead sorption and leaching from an Inceptisol sample amended with sugarcane vinasse. Sci. Agric. 2010, 67, 441–447. [Google Scholar] [CrossRef]
- Rolim, M.M.; Lyra, M.R.C.C.; Duarte, A.S.; Medeiros, P.R.F.; França e Silva, E.F.; Pedrosa, E.M.R. Influence of a vinasse-distributing lake on water quality of the groundwater. Rev. Ambient. Água 2013, 8, 155–171. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Silva, W.P.; Almeida, C.D.G.C.; Rolim, M.M.; Silva, E.F.F.; Pedrosa, E.M.R.; Silva, V.G.F. Monitoring of groundwater salinity in lowland under sugarcane cultivation fertigated with vinasse. Rev. Bras. Eng. Agríc. Ambient. 2014, 18, 394–401. (In Portuguese) [Google Scholar] [CrossRef] [Green Version]
- Tasso, L.G., Jr.; Marques, M.O.; Franco, A.; Nogueira, G.A.; Nobile, F.O.; Camilotti, F.; Silvam, A.R. Yield and quality of sugar cane cultivated in sewage sludge; vinasse and mineral fertilization supplied soil. Eng. Agríc. 2007, 27, 276–283. (In Portuguese) [Google Scholar]
- Uyeda, C.A.; Miranda, J.H.; Duarte, S.N.; Medeiros, P.R.F.; Dias, C.T.S. Influence of vinasse application in hydraulic conductivity of three soils. Eng. Agríc. 2013, 33, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Zolin, C.A.; Paulino, J.; Bertonha, A.; Freitas, P.S.L.; Folegatti, M.V. Exploratory study of the stillage use along the time. I. Characteristics of the soil. Rev. Bras. Eng. Agríc. Ambient. 2011, 15, 22–28. (In Portuguese) [Google Scholar] [CrossRef]
- Kadioğlu, A.; Algur, O.F. Tests of media with vinasse for Chlamydomonas reinhardii for possible reduction in vinasse pollution. Bioresour. Technol. 1992, 42, 1–5. [Google Scholar] [CrossRef]
- Marques, S.S.I.; Nascimento, I.A.; Almeida, P.F.; Chinalia, F.A. Growth of Chlorella vulgaris on sugarcane vinasse: The effect of anaerobic digestion pretreatment. Appl. Biochem. Biotechnol. 2013, 171, 1933–1943. [Google Scholar] [CrossRef]
- Santos, R.R.; Araújo, O.Q.F.; Medeiros, J.L.; Chaloub, R.M. Cultivation of Spirulina maxima in medium supplemented with sugarcane vinasse. Bioresour. Technol. 2016, 204, 38–48. [Google Scholar] [CrossRef]
- Candido, C.; Lombardi, A.T. The physiology of Chlorella vulgaris grown in conventional and biodigested treated vinasses. Algal Res. 2018, 30, 79–85. [Google Scholar] [CrossRef]
- Soto, M.F.; Diaz, C.A.; Zapata, A.M.; Higuita, J.C. BOD and COD removal in vinasse from sugarcane alcoholic distillation by Chlorella vulgaris: Environmental evaluation. Biochem. Eng. J. 2021, 176, 108191. [Google Scholar] [CrossRef]
- Pires, J.F.; Ferreira, G.M.R.; Reis, K.C.; Schwan, R.F.; Silva, C.F. Mixed yeasts inocula for simultaneous production of SCP and treatment of vinasse to reduce soil and fresh water pollution. J. Environ. Manag. 2016, 182, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.C.D.; Callieri, D.A.S.; Garro, O. Determination of the optimal conditions for the continuous culture of Candida utilis in sugarcane stillage. Appl. Microbiol. Biotechnol. 1987, 27, 100–104. [Google Scholar]
- Santos, J.F.; Canettieri, E.V.; Souza, S.M.A.; Rodrigues, R.C.L.B.; Martínez, E.C. Treatment of sugarcane vinasse from cachaça production for the obtainment of Candida utilis CCT 3469 biomass. Biochem. Eng. J. 2019, 148, 131–137. [Google Scholar] [CrossRef]
- Nunes, N.S.P.; Almeida, J.M.O.; Fonseca, G.G.; Carvalho, E.M. Clarification of sugarcane (Saccharum offcinarum) vinasse for microalgae cultivation. Bioresour. Technol. Rep. 2022, 19, 101125. [Google Scholar] [CrossRef]
- Kahraman, S.S.; Gurdal, I.H. Effect of synthetic and natural culture media on laccase production by white rot fungi. Bioresour. Technol. 2002, 82, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Colin, V.L.; Cortes, A.A.J.; Aparicio, J.D.; Amoroso, M.J. Potential application of a bioemulsifier-producing actinobacterium for treatment of vinasse. Chemosphere 2016, 144, 842–847. [Google Scholar] [CrossRef]
- Bastos, R.G.; Morais, D.V.; Volpi, M.P.C. Influence of solid moisture and bed height on cultivation of Aspergillus niger from sugarcane bagasse with vinasse. Braz. J. Chem. Eng. 2015, 32, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Menezes, E.G.T.; Alves, J.G.L.F.; Valeriano, C.; Guimarães, I.C. Physico-chemical and sensorial evaluation of sugarcane spirits produced using distillation residue. Braz. Arch. Biol. Technol. 2013, 56, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.C.; Lin, I.H. Production of acid protease using thin stillage from a rice-spirit distillery by Aspergillus niger. Enzyme Microb. Technol. 1998, 23, 397–402. [Google Scholar] [CrossRef]
- Hsieh, C.; Hsu, T.H.; Yang, F.C. Production of polysaccharides of Ganoderma lucidum (CCRC36021) by reusing thin stillage. Process Biochem. 2005, 40, 909–916. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Strait, M.; Wen, Z. Use of dry-milling derived thin stillage for producing eicosapentaenoic acid (EPA) by the fungus Pythium irregulare. Bioresour. Technol. 2012, 111, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.W.; Yang, Y.C.; Yu, Y.H. Using crude glycerol and thin stillage for the production of microbial lipids through the cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2012, 114, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Liu, R.H. Thin stillage supplementation greatly enhances bacterial cellulose production by Gluconacetobacter xylinus. Carbohydr. Polym. 2012, 90, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Liu, R.H. Cost-effective production of bacterial cellulose in static cultures using distillery wastewater. J. Biosci. Bioeng. 2013, 115, 284–290. [Google Scholar] [CrossRef]
- Khan, N.H.; Kang, T.S.; Grahame, D.A.S.; Haakensen, M.C.; Ratanapariyanuch, K.; Reaney, M.J.; Korber, D.R.; Tanaka, T. Isolation and characterization of novel 1;3-propanediol-producing Lactobacillus panis PM1 from bioethanol thin stillage. Appl. Microbiol. Biotechnol. 2013, 97, 417–428. [Google Scholar] [CrossRef]
- Wang, L.; Dong, X.; Jiang, H.; Li, G.; Zhang, M. Preparation of a novel carbon-based solid acid from cassava stillage residue and its use for the esterification of free fatty acids in waste cooking oil. Bioresour. Technol. 2014, 158, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Djukić-Vuković, A.P.; Mojović, L.V.; Semenčenko, V.V.; Radosavljević, M.M.; Pejin, J.D.; Kocić-Tanackov, S.D. Effective valorisation of distillery stillage by integrated production of lactic acid and high quality feed. Food Res. Int. 2015, 73, 75–80. [Google Scholar] [CrossRef]
- Kim, S.M.; Li, S.; Pan, S.C.; Ding, Y.; Basu, R.; van Egmond, P.; Singh, V. A whole stillage sieving process to recover fiber for cellulosic ethanol production. Ind. Crops Prod. 2016, 92, 271–276. [Google Scholar] [CrossRef]
- Ray, S.G.; Ghangrekar, M.M. Biodegradation kinetics of thin-stillage treatment by Aspergillus awamori and characterization of recovered chitosan. Appl. Microbiol. Biotechnol. 2016, 100, 1955–1965. [Google Scholar] [CrossRef]
- Pereira, T.J. Study of the Use of Vinasse in the Preparation of the Yeast and in the Alcoholic Fermentation. Master’s Thesis, University of Ribeirão Preto, Ribeirão Preto, Brazil, 2009. (In Portuguese). [Google Scholar]
- Madaleno, L.L.; Barros, V.G.; Kesserling, M.A.; Teixeira, J.R.; Duda, R.M.; Oliveira, R.A. The recycling of biodigested vinasse in an upflow anaerobic sludge blanket reactor is a feasible approach for the conservation of freshwater in the biofuel ethanol industry. J. Clean. Prod. 2020, 262, 121196. [Google Scholar] [CrossRef]
- Bialas, W.; Szymanowska, D.; Grajek, W. Fuel ethanol production from granular corn starch using Saccharomyces cerevisiae in a long term repeated SSF process with full stillage recycling. Bioresour. Technol. 2010, 101, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, W.R.; Westby, C.A. Processing cereal grains; thin stillage; and cheese whey to fuel ethanol in a farm-scale plant. Biomass 1988, 15, 25–43. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.H.; Liu, P.; Cao, H.S.; Mao, Z.G. Reusing a mixture of anaerobic digestion effluent and thin stillage for cassava ethanol production. J. Clean. Prod. 2014, 75, 57–63. [Google Scholar] [CrossRef]
- Zi, L.H.; Liu, C.G.; Xin, C.B.; Bai, F.W. Stillage backset and its impact on ethanol fermentation by the flocculating yeast. Process Biochem. 2013, 48, 753–758. [Google Scholar] [CrossRef]
- Gurgel, M.N.A.; Correa, S.T.R.; Dourado Neto, D.; Paula Júnior, D.R. Technology for sugarcane agroindustry waste reuse as granulated organomineral fertilizer. Eng. Agríc. 2015, 35, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Nandy, T.; Shastry, S.; Kaul, S.N. Wastewater management in a cane molasses distillery involving bioresource recovery. J. Environ. Manag. 2002, 65, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.; Silva, A.L.L.; Costa, J.L.; Scheidt, G.N.; Novak, A.C.; Sydney, E.B.; Soccol, C.R. Development of a vinasse nutritive solution for hydroponics. J. Environ. Manag. 2013, 114, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Tan, C.K.; Garwood, R.; Thai, S.M. Vinasse—A potential biofuel—Cofiring with coal in a fluidised combustor. Fuel 2015, 158, 1006–1015. [Google Scholar] [CrossRef]
- Cortez, L.A.B.; Pérez, L.E.B. Experiences on vinasse disposal: Part III: Combustion of vinasse–#6 fuel oil emulsions. Braz. J. Chem. Eng. 1997, 14. [Google Scholar] [CrossRef]
- Crivelaro, S.H.R.; Mariano, A.P.; Furlan, L.T.; Gonçalves, R.A.; Seabra, P.N.; Angelis, D.F. Evaluation of the use of vinasse as a biostimulation agent for the biodegradation of oily sludge in soil. Braz. Arch. Biol. Technol. 2010, 53, 1217–1224. [Google Scholar] [CrossRef] [Green Version]
- Mariano, A.P.; Crivelaro, S.H.R.; Angelis, D.F.; Bonotto, D.M. The use of vinasse as an amendment to ex-situ bioremediation of soil and groundwater contaminated with diesel oil. Braz. Arch. Biol. Technol. 2009, 52, 1043–1055. [Google Scholar] [CrossRef]
- Tamashiro, J.R.; Kinoshita, A.; Silva, L.H.P.; Paiva, F.F.G.; Antunes, P.A.; Simões, R.D. Compressive resistance of concrete produced with recycled concrete aggregate and sugarcane vinasse waste-water. Clean. Eng. Technol. 2022, 6, 100362. [Google Scholar] [CrossRef]
- Carpanez, T.G.; Moreira, V.R.; Assis, I.R.; Amaral, M.C.S. Sugarcane vinasse as organi-mineral fertilizers feedstock: Opportunities and environmental risks. Sci. Total Environ. 2022, 832, 154998. [Google Scholar] [CrossRef] [PubMed]
- Madejón, E.; López, R.; Murillo, J.M.; Cabrera, F. Agricultural use of three (sugar-beet) vinasse composts: Effect on crops and chemical properties of a Cambisol soil in the Guadalquivir river valley (SW Spain). Agr. Ecosyst. Environ. 2001, 84, 55–65. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L. Effects on two beet vinasse forms on soil physical properties and soil loss. Catena 2006, 68, 41–50. [Google Scholar] [CrossRef]
- Tejada, M.; Garcia, C.; Gonzalez, J.L.; Hernandez, M.T. Organic amendment based on fresh and composted beet vinasses: Influence on soil properties and wheat yield. Soil Sci. Soc. Am. J. 2006, 70, 900–908. [Google Scholar] [CrossRef]
- Cruz, L.F.L.S.; Duarte, C.G.; Malheiros, T.F.; Pires, E.C. Technical, economic and environmental viability analysis of the current vinasse use: Ferti-irrigation, concentration and bio-digestion. Braz. J. Environ. Sci. RBCIAMB 2013, 29, 111–127. (In Portuguese) [Google Scholar]
- Lameloise, M.L.; Gavach, M.; Bouix, M.; Fargues, C. Combining reverse osmosis and ion-exchange allows beet distillery condensates to be recycled as fermentable dilution water. Desalination 2015, 363, 75–81. [Google Scholar] [CrossRef]
- Peiter, F.S.; Hankins, N.P.; Pires, E.C. Evaluation of concentration technologies in the design of biorefineries for the recovery or resources from vinasse. Water Res. 2019, 157, 483–497. [Google Scholar] [CrossRef]
- Annachhatre, A.P. Anaerobic treatment of industrial wastewaters. Resour. Conserv. Recy. 1996, 16, 161–166. [Google Scholar] [CrossRef]
- Gujer, W.; Zehnder, A.J.B. Conversion processes in anaerobic digestion. Water Sci. Technol. 1983, 15, 127–167. [Google Scholar] [CrossRef]
- Khanal, S.K. Anaerobic Biotechnology for Bioenergy Production: Principles and Applications, 1st ed.; Blackwell Publishing: New Delhi, India, 2008. [Google Scholar]
- McCarty, P.L.; Smith, D.P. Anaerobic wastewater treatment. Environ. Sci. Technol. 1986, 20, 1200–1206. [Google Scholar] [CrossRef]
- Mosey, F.E. Mathematical modeling of the anaerobic digestion process: Regulatory mechanisms for the formation of short-chain volatile acids from glucose. Water Sci. Technol. 1983, 15, 209–232. [Google Scholar] [CrossRef]
- Stams, A.J.M. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Van Leeuwenhoek 1994, 66, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, K.V.; Balakrishnan, M.; Kansal, A.; Lata, K.; Kishore, V.V.N. State-of-the-art of anaerobic digestion technology for industrial wastewater treatment. Renew. Sustain. Energy Rev. 2000, 4, 135–156. [Google Scholar] [CrossRef]
- van Lier, J.B. High-rate anaerobic wastewater treatment: Diversifying from end-of-the-pipe treatment to resource-oriented conversion techniques. Water Sci. Technol. 2008, 57, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- van Lier, J.B.; van der Zee, F.P.; Frijters, C.T.M.J.; Ersahin, M.E. Celebrating 40 years anaerobic sludge bed reactors for industrial wastewater treatment. Rev. Environ. Sci. Biotechnol. 2015, 14, 681–702. [Google Scholar] [CrossRef]
- Deh Kiani, M.K.; Parsaee, M.; Ardebili, S.M.S.; Reyes, I.P.; Fuess, L.T.; Karimi, K. Different bioreactor configurations for biogas production from sugarcane vinasse: A comprehensive review. Biomass Bioenergy 2022, 161, 106446. [Google Scholar] [CrossRef]
- Moletta, R. Winery and distillery wastewater treatment by anaerobic digestion. Water Sci. Technol. 2005, 51, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.E.; Ollis, D.F. Biochemical Engineering Fundamentals, 2nd ed.; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Chernicharo, C.A.L. Anaerobic reactors, 1st ed.; IWA Publishing: London, UK, 2007. [Google Scholar]
- Speece, R.E. Anaerobic Biotechnology for Industrial Wastewaters; Archae Press: Nashville, TN, USA, 1996. [Google Scholar]
- Boncz, M.A.; Formagini, E.L.; Santos, L.S.; Marques, R.D.; Paulo, P.L. Application of urea dosing for alkalinity supply during anaerobic digestion of vinasse. Water Sci. Technol. 2012, 66, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; Araújo, M.M., Jr.; Garcia, M.L.; Zaiat, M. Designing full-scale biodigestion plants for the treatment of vinasse in sugarcane biorefineries: How phase separation and alkalinization impact biogas and electricity production costs? Chem. Eng. Res. Des. 2017, 119, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Godoi, L.A.G.; Damianovic, M.H.R.; Foresti, E. Sulfidogenesis interference on methane production from carbohydrate-rich wastewater. Water Sci. Technol. 2015, 72, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Lens, P.N.L.; Visser, A.; Janssen, A.J.H.; Hulshoff Pol, L.W.; Lettinga, G. Biotechnological treatment of sulfate-rich wastewaters. Crit. Rev. Environ. Sci. Technol. 1998, 28, 41–88. [Google Scholar] [CrossRef]
- Damianovic, M.H.R.Z.; Foresti, E. Dynamics of sulfidogenesis associated to methanogenesis in horizontal-flow anaerobic immobilized biomass reactor. Process Biochem. 2009, 44, 1050–1054. [Google Scholar] [CrossRef]
- Anzola-Rojas, M.P.; Zaiat, M.; De Wever, H. Improvement of hydrogen production via ethanol-type fermentation in an anaerobic down-flow structured bed reactor. Bioresour. Technol. 2016, 202, 42–49. [Google Scholar] [CrossRef]
- Saady, N.M.C. Homoacetogenesis during hydrogen production by mixed cultures dark fermentation. Int. J. Hydrogen Energy 2013, 38, 13172–13191. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nishimura, Y. Hydrogen production by fermentation using acetic acid and lactic acid. J. Biosci. Bioeng. 2007, 103, 236–241. [Google Scholar] [CrossRef]
- Seeliger, S.; Janssen, P.H.; Schink, B. Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol. Lett. 2002, 211, 65–70. [Google Scholar] [CrossRef]
- Thauer, R.K.; Jungermann, K.; Decker, K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 1977, 41, 100–180. [Google Scholar] [CrossRef]
- Zhou, J.; Xing, J. Effect of electron donors on the performance of haloalkaliphilic sulfate-reducing bioreactors for flue gas treatment and microbial degradation patterns related to sulfate reduction of different electron donors. Biochem. Eng. J. 2015, 96, 14–22. [Google Scholar] [CrossRef]
- Bertolino, S.M.; Melgaço, L.A.; Sá, R.G.; Leão, V.A. Comparing lactate and glycerol as single-electron donor for sulfate reduction in fluidized bed reactors. Biodegradation 2014, 25, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.J.C.B.; Rocha, B.B.M.; Viana, C.E.; Toledo, A.C. Utilization of vinasse effluents from an anaerobic reactor. Water Sci. Technol. 1986, 18, 135–141. [Google Scholar] [CrossRef]
- Russo, C.; Sant’Anna, G.L., Jr.; Pereira, S.E.C. An anaerobic filter applied to the treatment of distillery wastewaters. Agric. Wastes 1985, 14, 301–313. [Google Scholar] [CrossRef]
- Barros, V.G.; Duda, R.M.; Oliveira, R.A. Biomethane production from vinasse in upflow anaerobic sludge blanket reactors inoculated with granular sludge. Braz. J. Microbiol. 2016, 47, 628–639. [Google Scholar] [CrossRef] [Green Version]
- Mota, V.T.; Santos, F.S.; Amaral, M.C.S. Two-stage anaerobic membrane bioreactor for the treatment of sugarcane vinasse: Assessment on biological activity and filtration performance. Bioresour. Technol. 2013, 146, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Vuitik, G.A.; Fuess, L.T.; Del Nery, V.; Bañares-Alcántara, R.; Pires, E.C. Effects of recirculation in anaerobic baffled reactors. J. Water Process Eng. 2019, 28, 36–44. [Google Scholar] [CrossRef]
- Rocha, M.H.; Elia Neto, A.; Salomon, K.R.; Lora, E.E.S.; Venturini, O.J.; del Olmo, A.O.; Rambla, M.A.O. Residues from biofuel production: Vinasse and glycerin. In Biofuels; Lora, E.E.S., Venturini, O.J., Eds.; Interciência: Rio de Janeiro, Brazil, 2012; Volume 2, pp. 691–809. [Google Scholar]
- Acharya, B.K.; Mohana, S.; Madamwar, D. Anaerobic treatment of distillery spent wash—A study on upflow anaerobic fixed film bioreactor. Bioresour. Technol. 2008, 99, 4621–4626. [Google Scholar] [CrossRef]
- España-Gamboa, E.; Mijangos-Cortés, J.; Hernández-Zárate, G.; Maldonado, J.A.D.; Alzate-Gaviria, L. Methane production by treating vinasses from hydrous ethanol using a modified UASB reactor. Biotechnol. Biofuels. 2012, 5, 82. [Google Scholar] [CrossRef] [Green Version]
- Fernández, N.; Montalvo, S.; Borja, R.; Guerrero, L.; Sánchez, E.; Cortés, I.; Colmenarejo, M.F.; Travieso, L.; Raposo, F. Performance evaluation of an anaerobic fluidized bed reactor with natural zeolite as support material when treating high-strength distillery wastewater. Renew. Energy 2008, 33, 2458–2466. [Google Scholar] [CrossRef]
- Kumar, G.S.; Gupta, S.K.; Singh, G. Biodegration of distillery spent wash in anaerobic hybrid reactor. Water Res. 2007, 41, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Salomon, K.R.; Lora, E.E.S. Estimate of the electric energy generating potential for different sources of biogas in Brazil. Biomass Bioenergy 2009, 33, 1101–1107. [Google Scholar] [CrossRef]
- Janke, L.; Leite, A.F.; Batista, K.; Silva, W.; Nikolausz, M.; Nelles, M.; Stinner, W. Enhancing biogas production from vinasse in sugarcane biorefineries: Effects of urea and trace elements supplementation on process performance and stability. Bioresour. Technol. 2016, 217, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.P.; Santos, I.F.S.; Silva, A.P.M.; Barros, R.M.; Ribeiro, E.M. Vinasse biogas for energy generation in Brazil: An assessment of economic feasibility, energy potential and avoided CO2 emissions. J. Clean. Prod. 2017, 151, 260–271. [Google Scholar] [CrossRef] [Green Version]
- Fuess, L.T.; Garcia, M.L. Anaerobic digestion for enhanced bioenergy generation in ethanol biorefineries: Understanding the potentials of vinasse as a biofuel. In Bioenergy Systems for the Future: Prospects for Biofuels and Biohydrogen; Dalena, F., Basile, A., Rossi, C., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 149–183. [Google Scholar]
- Nadaleti, W.C.; Lourenço, V.A.; Belli Filho, P.B.; Santos, G.B.; Przybyla, G. National potential production of methane and electrical energy from sugarcane vinasse in Brazil: A thermo-economic analysis. J. Environ. Chem. Eng. 2020, 8, 103422. [Google Scholar] [CrossRef]
- Nogueira, C.E.C.; Souza, S.N.M.; Micuanski, V.C.; Azevedo, R.L. Exploring possibilities of energy insertion from vinasse biogas in the energy matrix of Paraná State, Brazil. Renew. Sustain. Energy Rev. 2015, 48, 300–305. [Google Scholar] [CrossRef]
- Pereira, I.Z.; Santos, I.F.S.; Barros, R.M.; Castro e Silva, H.L.; Tiago Filho, G.L.; Moni e Silva, A.P. Vinasse biogas energy and economic analysis in the state of São Paulo, Brazil. J. Clean. Prod. 2020, 260, 121018. [Google Scholar] [CrossRef]
- Rocha, M.H.; Lora, E.E.S.; Venturini, O.J.; Escobar, J.C.P.; Santos, J.J.C.S.; Moura, A.G. Use of the life cycle assessment (LCA) for comparison of the environmental performance of four alternatives for the treatment and disposal of bioethanol stillage. Int. Sugar J. 2010, 112, 611–622. [Google Scholar]
- Moraes, B.S.; Palacios-Bereche, R.; Martins, G.; Nebra, S.A.; Fuess, L.T.; Silva, A.J.; Clementino, W.S.; Bajay, S.V.; Manduca, P.C.; Lamparelli, R.A.; et al. Biogas production: Technologies and applications. In Biofuels and Biorefining: Current Technologies for Biomass Conversion; Castro, F.I.G., Gutiérrez-Antonio, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 215–282. [Google Scholar]
- Boddey, R.M.; Soares, L.H.B.; Alves, B.J.R.; Urquiaga, S. Bioethanol production in Brazil. In Biofuels; Solar; and Wind as Renewable Energy Systems; Pimentel, D., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 321–356. [Google Scholar]
- CGEE. Sustainability of Sugarcane Bioenergy—Updated Edition; CGEE: Brasília, Brazil, 2012; Available online: https://www.cgee.org.br/documents/10195/734063/Book_sustainabily_updated_3a_final06072012_9534.pdf/059bf982-ec47-4ce8-9ac1-dc53f093d4cf?version=1.4 (accessed on 27 September 2022).
- Khan, A.S.; Fox, R.W. Net energy analyses of ethanol production from sugarcane in Northeast Brazil. Biomass 1982, 2, 213–221. [Google Scholar] [CrossRef]
- Macedo, I.C. The sugar cane agro-industry—Its contribution to reducing CO2 emissions in Brazil. Biomass Bioenergy 1992, 3, 77–80. [Google Scholar] [CrossRef]
- Macedo, I.C. Greenhouse gas emissions and energy balances in bio-ethanol production and utilization in Brazil (1996). Biomass Bioenergy 1998, 14, 77–81. [Google Scholar] [CrossRef]
- Macedo, I.C.; Seabra, J.E.A.; Silva, J.E.A.R. Green house gases emissions in the production and use of ethanol from sugarcane in Brazil: The 2005/2006 averages and a prediction for 2020. Biomass Bioenergy 2008, 32, 582–595. [Google Scholar] [CrossRef]
- Oliveira, M.E.D.; Vaughan, B.E.; Rykiel, E.J., Jr. Ethanol as fuel: Energy; carbon dioxide balances; and ecological footprint. Bioscience 2005, 55, 593–602. [Google Scholar] [CrossRef]
- Turdera, M.V. Energy balance; forecasting of bioelectricity generation and greenhouse gas emission balance in the ethanol production at sugarcane mills in the state of Mato Grosso do Sul. Renew. Sustain. Energy Rev. 2013, 19, 582–588. [Google Scholar] [CrossRef]
- EPE. 2021 Brazilian Energy Balance—Year 2020; Final Report; EPE: Rio de Janeiro, Brazil, 2021. Available online: https://www.epe.gov.br/pt/publicacoes-dados-abertos/publicacoes/balanco-energetico-nacional-2021 (accessed on 27 September 2022).
- Ke, S.; Shi, Z.; Fang, H.H.P. Applications of two-phase anaerobic degradation in industrial wastewater treatment. Int. J. Environ. Pollut. 2005, 23, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Hallenbeck, P.C. Fermentative hydrogen production: Principles, progress, and prognosis. Int. J. Hydrogen Energy 2009, 34, 7379–7389. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Lu, Y.; Wu, X.; Wang, L.; Wang, L.; Han, B.; Xing, X.H. States and challenges for high-value biohythane production from waste biomass by dark fermentation technology. Bioresour. Technol. 2013, 135, 292–303. [Google Scholar] [CrossRef]
- Sarma, S.J.; Pachapur, V.; Kaur Brar, S.; Le Bihan, Y.; Buelna, G. Hydrogen biorefinery: Potential utilization of the liquid waste from fermentative hydrogen production. Renew. Sustain. Energy Rev. 2015, 50, 942–951. [Google Scholar] [CrossRef]
- Yu, B.; Shan, A.; Zhang, D.; Lou, Z.; Yuan, H.; Huang, X.; Zhu, N.; Hu, X. Dosing time of ferric chloride to disinhibit the excessive volatile fatty acids in sludge thermophilic anaerobic digestion system. Bioresour. Technol. 2015, 189, 154–161. [Google Scholar] [CrossRef]
- Holl, E.; Steinbrenner, J.; Merkle, W.; Krümpel, J.; Lansing, S.; Baier, U.; Oechsner, H.; Lemmer, A. Two-stage anaerobic digestion: State of technology and perspective roles in future energy systems. Bioresour. Technol. 2022, 360, 127633. [Google Scholar] [CrossRef]
- Albanez, R.; Lovato, G.; Zaiat, M.; Ratusznei, S.M.; Rodrigues, J.A.D. Optimization, metabolic pathways modeling and scale-up estimative of an AnSBBR applied to biohydrogen production by co-digestion of vinasse and molasses. Int. J. Hydrogen Energy 2016, 41, 20473–20484. [Google Scholar] [CrossRef]
- Ferraz, A.D.N., Jr.; Wenzel, J.; Etchebehere, C.; Zaiat, M. Effect of organic loading rate on hydrogen production from sugarcane vinasse in thermophilic acidogenic packed bed reactors. Int. J. Hydrogen Energy 2014, 39, 16852–16862. [Google Scholar]
- Ferraz, A.D.N., Jr.; Etchebehere, C.; Zaiat, M. High organic loading rate on thermophilic hydrogen production and metagenomic study at an anaerobic packed-bed reactor treating a residual liquid stream of a Brazilian biorefinery. Bioresour. Technol. 2015, 186, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.D.N., Jr.; Etchebehere, C.; Zaiat, M. Mesophilic hydrogen production in acidogenic packed-bed reactors (APBR) using raw sugarcane vinasse as substrate: Influence of support materials. Anaerobe 2015, 34, 94–105. [Google Scholar]
- Lazaro, C.Z.; Perna, V.; Etchebehere, C.; Varesche, M.B.A. Sugarcane vinasse as substrate for fermentative hydrogen production: The effects of temperature and substrate concentration. Int. J. Hydrogen Energy 2014, 39, 6407–6418. [Google Scholar] [CrossRef]
- Lazaro, C.Z.; Varesche, M.B.A.; Silva, E.L. Sequential fermentative and phototrophic system for hydrogen production: An approach for Brazilian alcohol distillery wastewater. Int. J. Hydrogen Energy 2015, 40, 9642–9655. [Google Scholar] [CrossRef]
- Bernal, A.P.; Menezes, C.A.; Silva, E.L. A new side-looking at the dark fermentation of sugarcane vinasse: Improving the carboxylates production in mesophilic EGSB by selection of the hydraulic retention time and substrate concentration. Int. J. Hydrogen Energy 2021, 46, 12758–12770. [Google Scholar] [CrossRef]
- Niz, M.Y.K.; Etchelet, I.; Fuentes, L.; Etchebehere, C.; Zaiat, M. Extreme thermophilic condition: An alternative for long-term biohydrogen production from sugarcane vinasse. Int. J. Hydrogen Energy 2019, 44, 22876–22887. [Google Scholar] [CrossRef]
- Ramos, L.R.; Silva, E.L. Continuous hydrogen production from agricultural wastewaters at thermophilic and hyperthermophilic temperatures. Appl. Biochem. Biotechnol. 2017, 182, 846–869. [Google Scholar] [CrossRef]
- Ramos, L.R.; Silva, E.L. Thermophilic hydrogen and methane production from sugarcane stillage in two-stage anaerobic fluidized bed reactors. Int. J. Hydrogen Energy 2020, 45, 5239–5251. [Google Scholar] [CrossRef]
- Rego, G.C.; Ferreira, T.B.; Ramos, L.R.; Menezes, C.A.; Soares, L.A.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Bioconversion of pretreated sugarcane vinasse into hydrogen: New perspectives to solve one of the greatest issues of the sugarcane biorefinery. Biomass Conv. Bioref. 2022, 12, 5527–5541. [Google Scholar] [CrossRef]
- Reis, C.M.; Carosia, M.F.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Evaluation of hydrogen and methane production from sugarcane vinasse in an anaerobic fluidized bed reactor. Int. J. Hydrogen Energy 2015, 40, 8498–8509. [Google Scholar] [CrossRef]
- Santos, S.C.; Rosa, P.R.F.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Continuous thermophilic hydrogen production and microbial community analysis from anaerobic digestion of diluted sugar cane stillage. Int. J. Hydrogen Energy 2014, 39, 9000–9011. [Google Scholar] [CrossRef]
- Santos, S.C.; Rosa, P.R.F.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Hydrogen production from diluted and raw sugarcane vinasse under thermophilic anaerobic conditions. Int. J. Hydrogen Energy 2014, 39, 9599–9610. [Google Scholar] [CrossRef]
- Santos, S.C.; Rosa, P.R.F.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Organic loading rate impact on biohydrogen production and microbial communities at anaerobic fluidized thermophilic bed reactors treating sugarcane stillage. Bioresour. Technol. 2014, 159, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 1st ed.; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Pawar, S.S.; van Niel, E.W.J. Thermophilic biohydrogen production: How far are we? Appl. Microbiol. Biotechnol. 2013, 97, 7999–8009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthic, P.; Shiny, J. Comparison and limitations of biohydrogen production processes. Res. J. Biotechnol. 2012, 7, 59–71. [Google Scholar]
- Bartels, J.R.; Pate, M.B.; Olson, N.K. An economic survey of hydrogen production from conventional and alternative energy sources. Int. J. Hydrogen Energy 2010, 35, 8371–8384. [Google Scholar] [CrossRef]
- Chandra, R.; Mohan, S.V. Enhanced bio-hydrogenesis by co-culturing photosynthetic bacteria with acidogenic process: Augmented dark-photo fermentative hybrid system to regulate volatile fatty acid inhibition. Int. J. Hydrogen Energy 2014, 39, 7604–7615. [Google Scholar] [CrossRef]
- Fuess, L.T.; Ferraz, A.D.N., Jr.; Machado, C.B.; Zaiat, M. Temporal dynamics and metabolic correlation between lactate-producing and hydrogen-producing bacteria in sugarcane vinasse dark fermentation: The key role of lactate. Bioresour. Technol. 2018, 247, 426–433. [Google Scholar] [CrossRef]
- Cavinato, C.; Giuliano, A.; Bolzonella, D.; Pavan, P.; Cecchi, F. Bio-hythane production from food waste by dark fermentation coupled with anaerobic digestion process: A long-term pilot scale experience. Int. J. Hydrogen Energy 2012, 37, 11549–11555. [Google Scholar] [CrossRef]
- Noori, M.T.; Min, B. Fundamentals and recent progress in bioelectrochemical system-assisted biohythane production. Bioresour. Technol. 2022, 361, 127641. [Google Scholar] [CrossRef] [PubMed]
- Çeper, B.A. Use of hydrogen-methane blends in internal combustion engines. In Hydrogen Energy—Challenges and Perspectives; Minic, D., Ed.; InTech: Rijeka, Croatia, 2012; pp. 175–200. [Google Scholar]
- Ma, F.; Naeve, N.; Wang, M.; Jiang, L.; Chen, R.; Zhao, S. Hydrogen-enriched compressed natural gas as a fuel for engines. In Natural Gas; Potocnik, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 307–332. [Google Scholar]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Johansson, S.; Boe, K.; Xie, L.; Zhou, Q.; Angelidaki, I. Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor. Biotechnol. Bioeng. 2012, 109, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Dong, R. Recent progress towards in-situ biogas upgrading Technologies. Sci. Total Environ. 2021, 800, 149667. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lin, C.Y.; Chang, J.S. Biohydrogen production: Current perspectives and the way forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Silva, A.J.; Pozzi, E.; Foresti, E.; Zaiat, M. The influence of the buffering capacity on the production of organic acids and alcohols from wastewater in anaerobic reactor. Appl. Biochem. Biotechnol. 2014, 175, 2258–2265. [Google Scholar] [CrossRef]
- Eng, F.; Fuess, L.T.; Bovio-Winkler, P.; Etchebehere, C.; Sakamoto, I.K.; Zaiat, M. Optimization of volatile fatty acid production by sugarcane vinasse dark fermentation using a response surface methodology. Links between performance and microbial community composition. Sustain. Energy Technol. Assess. 2022, 53, 102764. [Google Scholar]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Lyberatos, G. Influence of pH on fermentative hydrogen production from sweet sorghum extract. Int. J. Hydrogen Energy 2010, 35, 1921–1928. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Effect of pH and retention time on volatile fatty acids production during mixed culture fermentation. Bioresour. Technol. 2015, 190, 274–280. [Google Scholar] [CrossRef]
- Xie, L.; Liu, H.; Chen, Y.G.; Zhou, Q. pH-adjustment strategy for volatile fatty acid production from high-strength wastewater for biological nutrient removal. Water Sci. Technol. 2014, 69, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Sjöblom, M.; Matsakas, L.; Christakopoulos, P.; Rova, U. Production of butyric acid by Clostridium tyrobutyricum (ATCC25755) using sweet sorghum stalks and beet molasses. Ind. Crops Prod. 2015, 74, 535–544. [Google Scholar] [CrossRef]
- Regestein, L.; Doerr, E.W.; Staaden, A.; Rehmann, L. Impact of butyric acid on butanol formation by Clostridium pasteurianum. Bioresour. Technol. 2015, 196, 153–159. [Google Scholar] [CrossRef]
- Pereira, L.G.; Dias, M.O.S.; Mariano, A.P.; Maciel Filho, R.; Bonomi, A. Economic and environmental assessment of n-butanol production in an integrated first and second generation sugarcane biorefinery: Fermentative versus catalytic routes. Appl. Energy 2015, 160, 120–131. [Google Scholar] [CrossRef]
- Vieira, C.F.S.; Sia, A.D.; Maugeri Filho, F.; Maciel Filho, R.; Mariano, A.P. Isopropanol-butanol-ethanol production by cell-immobilized vacuum fermentation. Bioresour. Technol. 2022, 344, 126313. [Google Scholar] [CrossRef] [PubMed]
- Zetty-Arenas, A.M.; Tovar, L.P.; Alves, R.F.; Mariano, A.P.; van Gulik, W.; Maciel Filho, R.; Freitas, S. Co-fermentation of sugarcane bagasse hydrolysate and molasses Clostridium saccharoperbutylacetonicum: Effect on sugar consumption and butanol production. Ind. Crops Prod. 2021, 167, 113512. [Google Scholar] [CrossRef]
- Jang, Y.S.; Im, J.A.; Choi, S.Y.; Lee, J.I.; Lee, S.Y. Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity. Metab. Eng. 2014, 23, 165–174. [Google Scholar] [CrossRef]
- Sydney, E.B.; Larroche, C.; Novak, A.C.; Nouaille, R.; Sarma, S.J.; Kaur Brar, S.; Letti, L.A.J.; Soccol, V.T.; Soccol, C.R. Economic process to produce biohydrogen and volatile fatty acids by a mixed culture using vinasse from sugarcane ethanol industry as nutrient source. Bioresour. Technol. 2014, 159, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Fuess, L.T.; Santos, G.M.; Delforno, T.P.; Moraes, B.S.; Silva, A.J. Biochemical butyrate production via dark fermentation as an energetically efficient alternative management approach for vinasse in sugarcane biorefineries. Renew. Energy 2020, 158, 3–12. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef]
- Amulya, K.; Reddy, M.V.; Mohan, S.V. Acidogenic spent wash valorization through polyhydroxyalkanoate (PHA) synthesis coupled with fermentative biohydrogen production. Bioresour. Technol. 2014, 158, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Marang, L.; Jiang, Y.; van Loosdrecht, M.C.M.; Kleerebezem, R. Butyrate as preferred substrate for polyhydroxybutyrate production. Bioresour. Technol. 2013, 142, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Nikhil, G.N.; Mohan, S.V.; Swamy, Y.V.; Sarma, P.N. Pseudomonas otitidis as a potential biocatalyst for polyhydroxyalkanoates (PHA) synthesis using synthetic wastewater and acidogenic effluents. Bioresour. Technol. 2012, 123, 471–479. [Google Scholar] [CrossRef]
- Liu, B.; Wen, Q.; Huang, L.; Chen, Z.; Lin, X.; Liu, S. Insights into integration of polyhydroxyalkanoates (PHAs) production into wastewater treatment: Comparison of different electron acceptors on system function and PHA-producer enrichment. Chem. Eng. J. 2023, 451, 138631. [Google Scholar] [CrossRef]
- Nonato, R.V.; Mantelatto, P.E.; Rossell, C.E.V. Integrated production of biodegradable plastic; sugar and ethanol. Appl. Microbiol. Biotechnol. 2001, 57, 1–5. [Google Scholar]
- Radivojevic, J.; Skaro, S.; Senerovic, L.; Vasiljevic, B.; Guzik, M.; Kenny, S.T.; Maslak, V.; Nikodinovic-Runic, J.; O’Connor, K.E. Polyhydroxyalkanoate-based 3-hydroxyoctanoic acid and its derivatives as a platform of bioactive compounds. Appl. Microbiol. Biotechnol. 2016, 100, 161–172. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Bordel, S.; Santos-Beneit, F.; Martínez-Mendoza, L.J.; Börner, R.A.; Börner, T.; Muñoz, R. Biodegradation of bioplastics under aerobic and anaerobic aqueous conditions: Kinetics, carbon fate and particle size effect. Bioresour. Technol. 2022, 344, 126265. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Börner, R.A.; Börner, T.; Muñoz, R. Production of volatile fatty acids (VFAs) from five commercial bioplastics via acidogenic fermentation. Bioresour. Technol. 2022, 360, 127655. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Reddy, M.V.; Subhash, G.V.; Sarma, P.N. Fermentative effluents from hydrogen producing bioreactor as substrate for poly(β-OH) butyrate production with simultaneous treatment: An integrated approach. Bioresour. Technol. 2010, 101, 9382–9386. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Eiroa, M.; Torres, C.; Nunes, B.R.; Reis, M.A.M. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 2007, 130, 411–421. [Google Scholar] [CrossRef]
- Oliveira, G.H.D.; Niz, M.Y.K.; Zaiat, M.; Rodrigues, J.A.D. Effects of organic loading rate on polyhydroxyalkanoate production from sugarcane stillage by mixed microbial cultures. Appl. Biochem. Biotechnol. 2019, 189, 1039–1055. [Google Scholar] [CrossRef] [PubMed]
- Silverio, M.S.; Piccoli, R.A.M.; Reis, J.L.M.S.; Gomez, J.G.C.; Baptista, A.S. Techno-economic feasibility of P(3-hydroxybutyrate) bioprocess with concentrated sugarcane vinasse as carbon and minerals source: An experimental and in silico approach. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Bekatorou, A.; Dima, A.; Tsafrakidou, P.; Boura, K.; Lappa, K.; Kandylis, P.; Pissaridi, K.; Kanellaki, M.; Koutinas, A.A. Downstream extraction process development for recovery of organic acids from a fermentation broth. Bioresour. Technol. 2016, 220, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.M.; Gonçalves, L.M.D.; Carrondo, M.J.T. Sulphate removal in acidogenic phase anaerobic digestion. Environ. Technol. 1988, 9, 775–784. [Google Scholar] [CrossRef]
- Reis, M.A.M.; Lemos, P.C.; Martins, M.J.; Costa, P.C.; Gonçalves, L.M.D.; Carrondo, M.J.T. Influence of sulfates and operational parameters on volatile fatty acids concentration profile in acidogenic phase. Bioprocess Eng. 1991, 6, 145–151. [Google Scholar] [CrossRef]
- Piffer, M.A.; Oliveira, C.A.; Bovio-Winkler, P.; Eng, F.; Etchebehere, C.; Zaiat, M.; Nascimento, C.A.O.; Fuess, L.T. Sulfate- and pH-driven metabolic flexibility in sugarcane vinasse dark fermentation stimulates biohydrogen evolution, sulfidogenesis or homoacetogenesis. Int. J. Hydrogen Energy 2022, 47, 31202–31222. [Google Scholar] [CrossRef]
- Junqueira, T.L.; Moraes, B.; Gouveia, V.L.R.; Chagas, M.F.; Morais, E.R.; Watanabe, M.D.B.; Zaiat, M.; Bonomi, A. Use of VSB to plan research programs and public policies. In Virtual Biorefinery: An Optimization Strategy for Renewable Carbon Valorization; Bonomi, A., Cavalett, O., Cunha, M.P., Lima, M.A.P., Eds.; Springer: London, UK, 2016; pp. 257–282. [Google Scholar]
- Souza, S.N.M.; Borsoi, A.; Santos, R.F.; Secco, D.; Frigo, E.P.; Silva, M.J. Production potential of biogas in sugar and ethanol plants for use in urban buses in Brazil. J. Food Agric. Environ. 2012, 10, 908–910. [Google Scholar]
- Wilkie, A.C. Biomethane from biomass, biowaste and biofuels. In Bioenergy; Wall, J.D., Harwood, C.S., Demain, A., Eds.; ASM Press: Washington, DC, USA, 2008; pp. 195–205. [Google Scholar]
- Fuess, L.T.; Zaiat, M.; Nascimento, C.A.O. Can biogas-producing sugarcane biorefineries techno-economically outperform conventional ethanol production? Deciphering the way towards maximum profitability. Energy Convers. Manag. 2022, 254, 115206. [Google Scholar] [CrossRef]
- ANP. Resolution ANP n. 685, 29 June 2017. Brasília, DF, Brazil. 2017. Available online: https://atosoficiais.com.br/anp/resolucao-n-685-2017-estabelece-as-regras-para-aprovacao-do-controle-da-qualidade-e-a-especificacao-do-biometano-oriundo-de-aterros-sanitarios-e-de-estacoes-de-tratamento-de-esgoto-destinado-ao-uso-veicular-e-as-instalacoes-residenciais-industriais-e-comerciais-a-ser-comercializado-em-todo-o-territorio-nacional?origin=instituicao&q=685/2017 (accessed on 27 September 2022).
- Barrera, E.L.; Rosa, E.; Spanjers, H.; Romero, O.; De Meester, S.; Dewulf, J. A comparative assessment of anaerobic digestion power plants as alternative to lagoons for vinasse treatment: Life cycle assessment and exergy analysis. J. Clean. Prod. 2016, 113, 459–471. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A critical review on the state-of-the-art of physical/chemical and biological technologies for an integral biogas upgrading. Rev. Environ. Sci. Biotechnol. 2015, 14, 727–759. [Google Scholar] [CrossRef] [Green Version]
- Leme, R.M.; Seabra, J.E.A. Technical-economic assessment of different biogas upgrading routes from vinasse anaerobic digestion in the Brazilian bioethanol industry. Energy 2017, 119, 754–766. [Google Scholar] [CrossRef]
- Allegue, L.B.; Hinge, J. Biogas Upgrading—Evaluation of Methods for H2S Removal; Danish Technological Institute: Taastrup, Denmark, 2014; Available online: https://www.teknologisk.dk/_/media/60599_Biogas%20upgrading.%20Evaluation%20of%20methods%20for%20H2S%20removal.pdf (accessed on 27 September 2022).
- Lebrero, R.; Toledo-Cervantes, A.; Muñoz, R.; Del Nery, V.; Foresti, E. Biogas upgrading from vinasse digesters: A comparison between an anoxic biotrickling filter and an algal-bacterial photobioreactor. J. Chem. Technol. Biotechnol. 2016, 91, 2488–2495. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.F.; Colturato, L.F.; Oliveira, J.P.; Leite, L.R.; Oliveira, G.; Chernicharo, C.A.; Araújo, J.C. Metagenomic analysis of a desulphurisation system used to treat biogas from vinasse methanisation. Bioresour. Technol. 2016, 205, 58–66. [Google Scholar] [CrossRef]
- Jespersen, N.D. Barron’s AP Chemistry, 4th ed.; Barron’s Education Series, Inc.: New York, NY, USA, 2007. [Google Scholar]
- US Geological Survey. Mineral Commodity Summaries 2022; US Geological Survey: Reston, VA, USA, 2022. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022.pdf (accessed on 19 September 2022).
- EPA. Catalog of CHP Technologies. US Environmental Protection Agency, Combined Heat and Power Partnership: Washington, DC, USA, 2017. Available online: https://www.epa.gov/sites/production/files/2015-07/documents/catalog_of_chp_technologies.pdf (accessed on 27 September 2022).
- Fuess, L.T.; Cruz, R.B.C.M.; Zaiat, M.; Nascimento, C.A.O. Diversifying the portfolio of sugarcane biorefineries: Anaerobic digestion as the core process for enhanced resource recovery. Renew. Sustain. Energy Rev. 2021, 147, 111246. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Aquino, G.S.; Heinrichs, R. Biofuel production from sugarcane: Various routes of harvesting energy from the crop. In Sugarcane Biofuels: Status, Potential, and Prospects of the Sweet Crop to Fuel the World; Khan, M.T., Khan, I.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 21–38. [Google Scholar]
- UN. Transforming Our World: The 2030 Agenda for Sustainable Development; A/RES/70/1; United Nations: New York, NY, USA, 2020; Available online: https://sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed on 27 September 2022).
- Ge, X.; Xu, F.; Li, Y. Solid-state anaerobic digestion of lignocellulosic biomass: Recent progress and perspectives. Bioresour. Technol. 2016, 205, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Xu, F.; Ge, X.; Li, Y. Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2015, 44, 824–834. [Google Scholar] [CrossRef]
- Adarme, O.F.H.; Baêta, B.E.L.; Gabriel Filho, J.B.; Gurgel, L.V.A.; Aquino, S.F. Use of anaerobic co-digestion as an alternative to add value to sugarcane biorefinery wastes. Bioresour. Technol. 2019, 287, 121443. [Google Scholar] [CrossRef]
- Barros, V.G.; Duda, R.M.; Vantini, J.S.; Omori, W.P.; Ferro, M.I.T.; Oliveira, R.A. Improved methane production from sugarcane vinasse with filter cake in thermophilic UASB reactors, with predominance of Methanothermobacter and Methanosarcina archaea and Thermotogae bacteria. Bioresour. Technol. 2017, 244, 371–381. [Google Scholar] [CrossRef]
- González, L.M.L.; Reyes, I.P.; Romero, O.R. Anaerobic co-digestion of sugarcane press mud with vinasse on methane yield. Waste Manag. 2017, 68, 139–145. [Google Scholar] [CrossRef]
- Volpi, M.P.C.; Fuess, L.T.; Moraes, B.S. Anaerobic co-digestion of residues in 1G2G sugarcane biorefineries for enhanced electricity and biomethane production. Bioresour. Technol. 2021, 330, 124999. [Google Scholar] [CrossRef]
- Anupama; Ravindra, P. Value-added food: Single cell protein. Biotechnol. Adv. 2000, 18, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jayaraman, S.; Singh, G.; Srinivasan, M.P. Single step peroxidase extraction and oxidation of highly concentrated ethanol and phenol aqueous solutions using supercritical carbon dioxide. J. Supercritic. Fluids 2016, 116, 209–214. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid. Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas, M.M.F. Vinasse Treatment in Anaerobic Sequencing Batch Reactor with Immobilized Biomass under Thermophilic and Mesophilic Conditions. Ph.D. Thesis, University of São Paulo, São Carlos, Brazil, 2006. (In Portuguese). [Google Scholar]
- Heijnen, J.J.; Mulder, A.; Enger, W.; Hoeks, F. Review on the application of anaerobic fluidized bed reactors in waste-water treatment. Chem. Eng. J. 1989, 41, B37–B50. [Google Scholar] [CrossRef]
- CONAB. Thermoelectric Generation from Sugarcane Bagasse Burning in Brazil: Performance Assessment of the 2009/2010 Harvest; CONAB: Brasília, Brazil, 2011. Available online: https://www.gov.br/agricultura/pt-br/assuntos/sustentabilidade/agroenergia/arquivos-termoeletrica-com-a-queima-do-bagaco-de-cana-de-acucar/termoeletrica-com-a-queima-do-bagaco-de-cana-de-acucar-no-brasil-safra-2009-2010.pdf (accessed on 23 September 2022). (In Portuguese)




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuess, L.T.; Lens, P.N.L.; Garcia, M.L.; Zaiat, M. Exploring Potentials for Bioresource and Bioenergy Recovery from Vinasse, the “New” Protagonist in Brazilian Sugarcane Biorefineries. Biomass 2022, 2, 374-411. https://doi.org/10.3390/biomass2040025
Fuess LT, Lens PNL, Garcia ML, Zaiat M. Exploring Potentials for Bioresource and Bioenergy Recovery from Vinasse, the “New” Protagonist in Brazilian Sugarcane Biorefineries. Biomass. 2022; 2(4):374-411. https://doi.org/10.3390/biomass2040025
Chicago/Turabian StyleFuess, Lucas T., Piet N. L. Lens, Marcelo L. Garcia, and Marcelo Zaiat. 2022. "Exploring Potentials for Bioresource and Bioenergy Recovery from Vinasse, the “New” Protagonist in Brazilian Sugarcane Biorefineries" Biomass 2, no. 4: 374-411. https://doi.org/10.3390/biomass2040025
APA StyleFuess, L. T., Lens, P. N. L., Garcia, M. L., & Zaiat, M. (2022). Exploring Potentials for Bioresource and Bioenergy Recovery from Vinasse, the “New” Protagonist in Brazilian Sugarcane Biorefineries. Biomass, 2(4), 374-411. https://doi.org/10.3390/biomass2040025