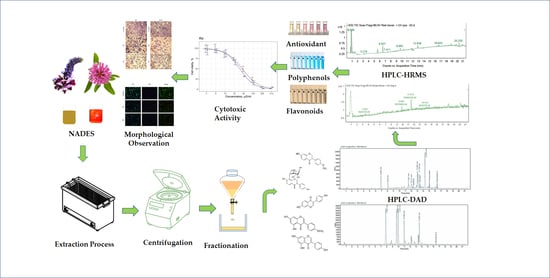
Cytotoxic Activity and Phytochemical Screening of Eco-Friendly Extracted Flavonoids from Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep and Trifolium pratense L. Flowers Using HPLC-DAD-MS/HRMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Equipment
2.2. Plant Materials
2.3. Preparation of Natural Deep Eutectic Solvents (NADESs)
2.4. NADES-Based Ultrasound-Assisted Extraction of KR and SM
2.5. Quantitative Determination of Isoflavones Using HPLC-DAD Method
2.6. Analysis of Isoflavones Using HPLC-ESI-HRMS Method
2.7. Assessment of Antioxidant Activity
2.8. Determination of Total Polyphenols
2.9. Determination of Total Flavonoids
2.10. Assessment of In Vitro Biological Activity
2.10.1. Culturing of Cell Lines
2.10.2. Cell Viability Assessment
2.10.3. Observation of Morphological Changes
- Dual AO/EB Fluorescent Staining
- Giemsa staining
2.11. Statistical Analysis
3. Results and Discussion
3.1. Identification and Quantification Isoflavones Present in KF and RC Using HPLC-DAD
3.2. Identification and Quantification of Bioactive Compounds Using HPLC-HRMS and MS/MS Analysis
3.2.1. Identification and Quantification of Bioactive Compounds in KF
3.2.2. Identification and Quantification of Bioactive Compounds in RC
3.3. Antioxidant Activity and Total Bioactive Compounds
3.4. Cytotoxicity Activity of KF and RC Extracts on Different Cancer Cell Lines
3.5. Morphological Observation
3.5.1. Acridine Orange and Ethidium Bromide
3.5.2. Giemsa Stain
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sushma, P.S.; Jamil, K.; Udaykumar, P.; Aldakheel, F.M.; Alduraywish, S.A.; Alali, B.H.; Mateen, A.; Syed, R. Analysis of CCND1 protein and circulatory antioxidant enzyme activity association in oral squamous cell carcinoma. Saudi J. Biol. Sci. 2021, 28, 6987–6991. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Zia ur, R.; Gurgul, A.; Youn, I.; Maldonado, A.; Wahid, F.; Che, C.-T.; Khan, T. UHPLC-MS/MS-GNPS based phytochemical investigation of Equisetum arvense L. and evaluation of cytotoxicity against human melanoma and ovarian cancer cells. Saudi J. Biol. Sci. 2022, 29, 103271. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.P.; Mencherini, T. Hazelnut (Corylus avellana L.) Shells Extract: Phenolic Composition, Antioxidant Effect and Cytotoxic Activity on Human Cancer Cell Lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef]
- Yock, T.I.; Yeap, B.Y.; Ebb, D.H.; Weyman, E.; Eaton, B.R.; Sherry, N.A.; Jones, R.M.; MacDonald, S.M.; Pulsifer, M.B.; Lavally, B.; et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: A phase 2 single-arm study. Lancet Oncol. 2016, 17, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Mechanism-based management for mucositis: Option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther. 2016, 9, 2007–2016. [Google Scholar] [CrossRef] [Green Version]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, S.; Tong, H.; Shi, S. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother. Res. 2018, 32, 384–394. [Google Scholar] [CrossRef]
- Patel, D.K. Biological Activities and Therapeutic Potential of Irigenin on Gastric, Lung, Prostate, Breast, and Endometrial Cancer: Pharmacological and Analytical Aspects. Curr. Cancer Ther. Rev. 2022, 18, 172–182. [Google Scholar] [CrossRef]
- Sekikawa, A.; Wharton, W.; Butts, B.; Veliky, C.V.; Garfein, J.; Li, J.; Goon, S.; Fort, A.; Li, M.; Hughes, T.M. Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia. Int. J. Mol. Sci. 2022, 23, 11921. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Gupta, K.; Biswas, S. A mechanistic insight of phytoestrogens used for Rheumatoid arthritis: An evidence-based review. Biomed. Pharmacother. 2021, 133, 111039. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidya, N.; Saravanan, K.; Halka, J.; Kowsalya, K.; Preetha, J.S.Y.; Gurusaravanan, P.; Radhakrishnan, R.; Nanthini, A.U.R.; Arun, M. An insight into in vitro strategies for bioproduction of isoflavones. Plant Biotechnol. Rep. 2021, 15, 717–740. [Google Scholar] [CrossRef]
- Aboushanab, S.A.; Khedr, S.M.; Gette, I.F.; Danilova, I.G.; Kolberg, N.A.; Ravishankar, G.A.; Ambati, R.R.; Kovaleva, E.G. Isoflavones derived from plant raw materials: Bioavailability, anti-cancer, anti-aging potentials, and microbiome modulation. Crit. Rev. Food Sci. Nutr. 2022, 63, 261–287. [Google Scholar] [CrossRef]
- Yu, Y.; Pauli, G.F.; Huang, L.; Gan, L.-S.; van Breemen, R.B.; Li, D.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N. Classification of Flavonoid Metabolomes via Data Mining and Quantification of Hydroxyl NMR Signals. Anal. Chem. 2020, 92, 4954–4962. [Google Scholar] [CrossRef]
- Duru, K.C.; Slesarev, G.P.; Aboushanab, S.A.; Kovalev, I.S.; Zeidler, D.M.; Kovaleva, E.G.; Bhat, R. An eco-friendly approach to enhance the extraction and recovery efficiency of isoflavones from kudzu roots and soy molasses wastes using ultrasound-assisted extraction with natural deep eutectic solvents (NADES). Ind. Crops Prod. 2022, 182, 114886. [Google Scholar] [CrossRef]
- Lu, J.; Xie, Y.; Tan, Y.; Qu, J.; Matsuda, H.; Yoshikawa, M.; Yuan, D. Simultaneous determination of isoflavones, saponins and flavones in Flos Puerariae by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Chem. Pharm. Bull. 2013, 61, 941–951. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, H.-W.; Lee, S.-J.; Kwon, R.H.; Na, H.; Kim, J.H.; Choi, Y.-M.; Yoon, H.; Kim, Y.-S.; Wee, C.-D.; et al. Comprehensive characterization of flavonoid derivatives in young leaves of core-collected soybean (Glycine max L.) cultivars based on high-resolution mass spectrometry. Sci. Rep. 2022, 12, 14678. [Google Scholar] [CrossRef]
- Qi, P.; Zhou, Q.-Q.; Lin, Z.-H.; Liu, J.; Cai, W.-y.; Mao, X.-W.; Jiang, J.-J. Qualitative screening and quantitative determination of multiclass water-soluble synthetic dyes in foodstuffs by liquid chromatography coupled to quadrupole Orbitrap mass spectrometry. Food Chem. 2021, 360, 129948. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.; Son, S.-R.; Kim, J.-Y.; Choi, J.-H.; Jang, D.S. Chemical Constituents of the Flowers of Pueraria lobata and Their Cytotoxic Properties. Plants 2022, 11, 1651. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Saravana Kumar, P.; Qiu, J.; Ran, Y.; Tan, X.; Zhao, R.; Ai, L.; Yuan, M.; Zhu, J.; He, M. Production of bioactive compounds from callus of Pueraria thomsonii Benth with promising cytotoxic and antibacterial activities. Arab. J. Chem. 2022, 15, 103854. [Google Scholar] [CrossRef]
- Khazaei, M.; Pazhouhi, M.; Khazaei, S. Evaluation of Hydro-Alcoholic Extract of Trifolium Pratens L. for Its Anti-Cancer Potential on U87MG Cell Line. Cell J. 2018, 20, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Aboushanab, S.A.; Shevyrin, V.A.; Slesarev, G.P.; Melekhin, V.V.; Shcheglova, A.V.; Makeev, O.G.; Kovaleva, E.G.; Kim, K.H. Antioxidant and Cytotoxic Activities of Kudzu Roots and Soy Molasses against Pediatric Tumors and Phytochemical Analysis of Isoflavones Using HPLC-DAD-ESI-HRMS. Plants 2022, 11, 741. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Noreen, H.; Semmar, N.; Farman, M.; McCullagh, J.S.O. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med. 2017, 10, 792–801. [Google Scholar] [CrossRef]
- Sumi, S.A.; Siraj, M.A.; Hossain, A.; Mia, M.S.; Afrin, S.; Rahman, M.M. Investigation of the key pharmacological activities of Ficus racemosa and analysis of its major bioactive polyphenols by HPLC-DAD. Evid. Based Complement. Alternat. Med. 2016, 2016, 3874516. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Wu, W.; Shen, S.; Fan, J.; Chang, Y.; Chen, S.; Ye, X. Evaluation of colorimetric methods for quantification of citrus flavonoids to avoid misuse. Anal. Methods 2018, 10, 2575–2587. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Guo, Q.; Yang, H.; Gao, W.; Li, P. pH-controlled reversible deep-eutectic solvent based enzyme system for simultaneous extraction and in-situ separation of isoflavones from Pueraria lobata. Sep. Purif. Technol. 2022, 292, 120992. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Row, K.H. Development of deep eutectic solvents for sustainable chemistry. J. Mol. Liq. 2022, 362, 119654. [Google Scholar] [CrossRef]
- Ivanović, M.; Grujić, D.; Cerar, J.; Islamčević Razboršek, M.; Topalić-Trivunović, L.; Savić, A.; Kočar, D.; Kolar, M. Extraction of Bioactive Metabolites from Achillea millefolium L. with Choline Chloride Based Natural Deep Eutectic Solvents: A Study of the Antioxidant and Antimicrobial Activity. Antioxidants 2022, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Ma, H.; Yang, F.; Duan, T.; Zhang, Y.; Dong, Y. Quantitative analysis of nine isoflavones in traditional Chinese medicines using mixed micellar liquid chromatography containing sodium dodecylsulfate/β-cyclodextrin supramolecular amphiphiles. J. Sep. Sci. 2021, 44, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.-O.; Ofosu, F.K.; Kim, N.-H.; Kilonzi, S.M.; Oh, D.-H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.; Liu, D.; Yang, M.; Wei, J. Analysis of Flavonoids in Dalbergia odorifera by Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry. Molecules 2020, 25, 389. [Google Scholar] [CrossRef] [Green Version]
- Tchoumtchoua, J.; Njamen, D.; Mbanya, J.C.; Skaltsounis, A.L.; Halabalaki, M. Structure-oriented UHPLC-LTQ Orbitrap-based approach as a dereplication strategy for the identification of isoflavonoids from Amphimas pterocarpoides crude extract. J. Mass Spectrom. 2013, 48, 561–575. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, Y.; Ye, J.; Cheng, T.; Liang, Y.; Zu, X.; Zhang, W. A comprehensive quality evaluation of Fuzi and its processed product through integration of UPLC-QTOF/MS combined MS/MS-based mass spectral molecular networking with multivariate statistical analysis and HPLC-MS/MS. J. Ethnopharmacol. 2021, 266, 113455. [Google Scholar] [CrossRef]
- Cai, T.; Guo, Z.-Q.; Xu, X.-Y.; Wu, Z.-J. Recent (2000–2015) developments in the analysis of minor unknown natural products based on characteristic fragment information using LC–MS. Mass Spectrom. Rev. 2018, 37, 202–216. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST20: Updates to the NIST Tandem and Electron Ionization Spectral Libraries. 2020. Available online: https://www.nist.gov/programs-projects/nist20-updates-nist-tandem-and-electron-ionization-spectral-libraries (accessed on 12 August 2022).
- METLIN Metabolomics Database and Library. Available online: https://www.agilent.com/en/product/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-application-solutions/life-sciences-solutions/metlin-metabolomics-database-library (accessed on 12 August 2022).
- MassBank of North America (MoNA). Available online: https://mona.fiehnlab.ucdavis.edu/ (accessed on 12 August 2022).
- MS-DIAL. Available online: http://prime.psc.riken.jp/compms/msdial/main.html#MSP (accessed on 12 August 2022).
- Schmidt, J. Negative ion electrospray high-resolution tandem mass spectrometry of polyphenols. J. Mass Spectrom. 2016, 51, 33–43. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, X.; Liu, Y.; Dong, G.; Hao, C.; Zhang, J.; Jiang, J.; Cheng, J.; Liu, A.; Chen, S. Identification and quantification of oligomeric proanthocyanidins, alkaloids, and flavonoids in lotus seeds: A potentially rich source of bioactive compounds. Food Chem. 2022, 379, 132124. [Google Scholar] [CrossRef] [PubMed]
- Franski, R.; Gierczyk, B.; Kozik, T.; Popenda, L.; Beszterda, M. Signals of diagnostic ions in the product ion spectra of [M—H](-) ions of methoxylated flavonoids. Rapid Commun. Mass Spectrom. 2019, 33, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Ma, Y.; Zhang, L.T.; Ren, Q. Identification and characterization of the chemical components of Iris tectorum Maxim. and evaluation of their nitric oxide inhibitory activity. Rapid Commun. Mass Spectrom. 2021, 35, e8959. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Zhao, C.; Xiao, H.; Fang, X.; Zheng, J. LC-Q-TOF-MS/MS detection of food flavonoids: Principle, methodology, and applications. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Liu, Q.; Pei, Y.; Wan, H.; Wang, M.; Liu, L.; Li, W.; Jin, J.; Liu, X. Chemical profiling and identification of Radix Cudramiae and their metabolites in rats using an ultra-high-performance liquid chromatography method coupled with time-of-flight tandem mass spectrometry. J. Sep. Sci. 2022, 1–13. [Google Scholar] [CrossRef]
- Han, J.; Xu, K.; Yan, Q.; Sui, W.; Zhang, H.; Wang, S.; Zhang, Z.; Wei, Z.; Han, F. Qualitative and quantitative evaluation of Flos Puerariae by using chemical fingerprint in combination with chemometrics method. J. Pharm. Anal. 2022, 12, 489–499. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Gercek, Y.C.; Celik, S.; Bayram, S. Screening of Plant Pollen Sources, Polyphenolic Compounds, Fatty Acids and Antioxidant/Antimicrobial Activity from Bee Pollen. Molecules 2022, 27, 117. [Google Scholar] [CrossRef]
- Huang, W.; Wen, F.; Ruan, S.; Gu, P.; Gu, S.; Song, S.; Zhou, J.; Li, Y.; Liu, J.; Shu, P. Integrating HPLC-Q-TOF-MS/MS, network pharmacology and experimental validation to decipher the chemical substances and mechanism of modified Gui-shao-liu-jun-zi decoction against gastric cancer. J. Tradit. Complement. Med. 2023, in press. [Google Scholar] [CrossRef]
- Ferrario, G.; Baron, G.; Gado, F.; Della Vedova, L.; Bombardelli, E.; Carini, M.; D’Amato, A.; Aldini, G.; Altomare, A. Polyphenols from Thinned Young Apples: HPLC-HRMS Profile and Evaluation of Their Anti-Oxidant and Anti-Inflammatory Activities by Proteomic Studies. Antioxidants 2022, 11, 1577. [Google Scholar] [CrossRef]
- Paillat, L.; Bordier, E.; Guepet, A.; Lima, J.; Boudah, S.; Murtaugh, A. Development and Validation of an On-Line HPLC-DAD-Antioxidant Assay (ORAC)/ESI-HRMS System to Identify Antioxidant Compounds in Complex Mixtures. J. Chromatogr. Sci. 2023. [Google Scholar] [CrossRef]
- Shi, S.; Ma, Y.; Zhang, Y.; Liu, L.; Liu, Q.; Peng, M.; Xiong, X. Systematic separation and purification of 18 antioxidants from Pueraria lobata flower using HSCCC target-guided by DPPH–HPLC experiment. Sep. Purif. Technol. 2012, 89, 225–233. [Google Scholar] [CrossRef]
- Thapa, P.; Kim, H.M.; Hong, J.-P.; Kim, R.; Paudel, S.B.; Choi, H.; Jang, D.S.; Nam, J.-W. Absolute Quantification of Isoflavones in the Flowers of Pueraria lobata by qHNMR. Plants 2022, 11, 548. [Google Scholar] [CrossRef]
- Aboushanab, S.; Shevyrin, V.; Kamel, M.; Kambele, J.; Kovaleva, E. Phytochemical screening and properties of botanical crude extracts and ethyl acetate fractions isolated by deep eutectic solvent. Chim. Technol. Acta 2022, 9, 20229404. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Li, R.; Zhang, H.; Sun, Y.; Jiang, L.; Wang, X.; Xiong, Y. Flos Puerariae-Semen Hoveniae medicinal pair extract ameliorates DSS-induced inflammatory bowel disease through regulating MAPK signaling and modulating gut microbiota composition. Front. Pharmacol. 2022, 13, 1034031. [Google Scholar] [CrossRef] [PubMed]
- Akbaribazm, M.; Khazaei, M.R.; Khazaei, M. Phytochemicals and antioxidant activity of alcoholic/hydroalcoholic extract of Trifolium pratense. Chin. Herb. Med. 2020, 12, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Malca-Garcia, G.R.; Liu, Y.; Dong, H.; Nikolić, D.; Friesen, J.B.; Lankin, D.C.; McAlpine, J.; Chen, S.-N.; Dietz, B.M.; Pauli, G.F. Auto-hydrolysis of red clover as “green” approach to (iso)flavonoid enriched products. Fitoterapia 2021, 152, 104878. [Google Scholar] [CrossRef]
- Vlaisavljević, S.; Kaurinović, B.; Popović, M.; Vasiljević, S. Profile of phenolic compounds in Trifolium pratense L. extracts at different growth stages and their biological activities. Int. J. Food Prop. 2017, 20, 3090–3101. [Google Scholar] [CrossRef] [Green Version]
- Lertpatipanpong, P.; Janpaijit, S.; Park, E.-Y.; Kim, C.-T.; Baek, S.J. Potential Anti-Diabetic Activity of Pueraria lobata Flower (Flos Puerariae) Extracts. Molecules 2020, 25, 3970. [Google Scholar] [CrossRef]
- Zawiślak, A.; Francik, R.; Francik, S.; Knapczyk, A. Impact of Drying Conditions on Antioxidant Activity of Red Clover (Trifolium pratense), Sweet Violet (Viola odorata) and Elderberry Flowers (Sambucus nigra). Materials 2022, 15, 3317. [Google Scholar] [CrossRef]
- Vlaisavljevic, S.; Kaurinovic, B.; Popovic, M.; Djurendic-Brenesel, M.; Vasiljevic, B.; Cvetkovic, D.; Vasiljevic, S. Trifolium pratense L. as a Potential Natural Antioxidant. Molecules 2014, 19, 713–725. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Li, S.; Yang, X.; Qin, Y.; Zhang, Y.; Liu, C. Screening and isolation of potential lactate dehydrogenase inhibitors from five Chinese medicinal herbs: Soybean, Radix pueraria, Flos pueraria, Rhizoma belamcandae, and Radix astragali. J. Sep. Sci. 2016, 39, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Xiang, J.; Wang, C.; Johnson, J.B.; Beta, T. Diverse polyphenol components contribute to antioxidant activity and hypoglycemic potential of mulberry varieties. LWT 2023, 173, 114308. [Google Scholar] [CrossRef]
- Song, E.J.; Kim, N.Y.; Heo, M.Y. Protective effect of korean medicinal plants on ethanol-induced cytotoxicity in HepG2 Cells. Nat. Prod. Sci. 2013, 19, 329–336. [Google Scholar]
- Bulugonda, R.K.; Kumar, K.A.; Gangappa, D.; Beeda, H.; Philip, G.H.; Muralidhara Rao, D.; Faisal, S.M. Mangiferin from Pueraria tuberosa reduces inflammation via inactivation of NLRP3 inflammasome. Sci. Rep. 2017, 7, 42683. [Google Scholar] [CrossRef] [Green Version]
- Zgórka, G.; Maciejewska-Turska, M.; Makuch-Kocka, A.; Plech, T. In Vitro Evaluation of the Antioxidant Activity and Chemopreventive Potential in Human Breast Cancer Cell Lines of the Standardized Extract Obtained from the Aerial Parts of Zigzag Clover (Trifolium medium L.). Pharmaceuticals 2022, 15, 699. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Budryn, G. The Effects of Trifolium pratense L. Sprouts’ Phenolic Compounds on Cell Growth and Migration of MDA-MB-231, MCF-7 and HUVEC Cells. Nutrients 2020, 12, 257. [Google Scholar] [CrossRef] [Green Version]
- Lateef, R.; Marhaba; Mandal, P.; Ansari, K.M.; Javed Akhtar, M.; Ahamed, M. Cytotoxicity and apoptosis induction of copper oxide-reduced graphene oxide nanocomposites in normal rat kidney cells. J. King Saud Univ.—Sci. 2023, 35, 102513. [Google Scholar] [CrossRef]
- Serpeloni, J.M.; Leal Specian, A.F.; Ribeiro, D.L.; Tuttis, K.; Vilegas, W.; Martínez-López, W.; Dokkedal, A.L.; Saldanha, L.L.; de Syllos Cólus, I.M.; Varanda, E.A. Antimutagenicity and induction of antioxidant defense by flavonoid rich extract of Myrcia bella Cambess. in normal and tumor gastric cells. J. Ethnopharmacol. 2015, 176, 345–355. [Google Scholar] [CrossRef] [Green Version]





| Parameters | KF (%) | RC (%) |
|---|---|---|
| Puerarin | nd | nd |
| Daidzein | 0.31 ± 0.007 a | 0.07 ± 0.006 a |
| Genistein | 0.19 ± 0.015 a | 0.19 ± 0.008 b |
| Formononetin | nd | 0.29 ± 0.007 b |
| Biochanin A | 0.09 ± 0.01 b | 0.19 ± 0.015 b |
| Sum | 0.59 ± 0.091 | 0.74 ± 0.007 |
| No. | Retention Time (tr, min) | Chemical Formula | Accurate Mass [M–H]− | Exact Mass [M–H]− | Error, ppm | MS/MS Fragment Ions | Peak Area (-EIC), % of Genistein | Compound Name |
|---|---|---|---|---|---|---|---|---|
| Isoflavones | ||||||||
| 1i | 11.47 | C15H10O5 | 269.0460 | 269.0455 | −1.77 | 239.0343, 223.0401, 180.0581, 159.0452, 135.0452, 133.0295, 117.0346, 107.0139, 91.0189, 63.0240 | 100.0 | Genistein * |
| 2i | 14.05 | C16H12O5 | 283.0618 | 283.0612 | −2.03 | 267.0299, 239.0350, 223.0401, 211.0401, 195.0452, 167.0502, 132.0217 | 30.0 | Biochanin A * |
| 3i | 10.40 | C15H10O4 | 253.0506 | 253.0506 | −0.03 | 223.0401, 208.0530, 195.0452, 180.0581, 133.0295, 132.0217, 91.0189 | 7.4 | Daidzein * |
| 4i | 7.91 | C21H20O10 | 431.0985 | 431.0984 | −0.39 | 311.0561, 269.0455, 268.0377, 267.0299, 239.0350, 223.0401, 211.0401, 133.0295, 132.0217 | 2.4 | Genistein-7-O-glucoside (genistin) ** |
| 5i | 9.87 | C15H10O6 | 285.0413 | 285.0405 | −2.78 | 151.0037, 133.0295, 123.0088, 117.0346 | 240.3 | 6- or 8-hydroxygenistein ** |
| 6i | 7.19 | C21H20O11 | 447.0940 | 447.0933 | −1.68 | 285.0405, 241.0506, 213.0557, 185.0608, 133.0295, 123.0088 | 12.3 | 6- or 8-hydroxygenistein-glucoside ** |
| 7i | 11.50 | C16H12O6 | 299.0566 | 299.0561 | −1.66 | 284.0326, 283.0248, 255.0299, 240.0428, 227.0350, 211.0401, 200.0479, 183.0452, 133.0295, 132.0217 | 344.6 | Tectorigenin ** |
| 8i | 8.22 | C16H12O6 | 299.0564 | 299.0561 | −1.02 | 283.0248, 255.0299, 240.0428, 227.0350, 211.0401, 200.0479, 183.0452, 133.0295, 59.0139 | 6.4 | Tectorigenin isomer 1 ** |
| 9i | 12.56 | C16H12O6 | 299.0570 | 299.0561 | −2.97 | 283.0248, 267.0299, 255.0299, 239.0350, 227.0350, 211.0401, 190.9986, 163.0037, 132.0217, 59.0139 | 53.0 | Tectorigenin isomer 2 ** |
| 10i | 8.22 | C22H22O11 | 461.1102 | 461.1089 | −2.73 | 299.0561, 284.0326, 283.0248, 255.0299, 240.0428, 227.0350, 211.0401, 200.0479, 183.0452, 136.9880 | 47.9 | Tectoridine |
| Flavones | ||||||||
| 1f | 12.28 | C15H10O5 | 269.0460 | 269.0455 | −1.56 | 225.0557, 151.0037, 149.0244, 117.0346, 107.0139 | 9.7 | Apigenin ** |
| 2f | 11.36 | C15H10O6 | 285.0410 | 285.0405 | −1.80 | 241.0506, 217.0506, 199.0401, 175.0401, 151.0037, 133.0295, 107.0139 | 18.6 | Luteolin ** |
| Flavonoles | ||||||||
| 1fl | 12.08 | C15H10O6 | 285.0411 | 285.0405 | −2.28 | 255.0299, 239.0350, 227.0350, 211.0401, 159.0452, 143.0502, 117.0346, 93.0346 | 24.4 | Kaempferol ** |
| 2fl | 6.45 | C15H12O6 | 287.0563 | 287.0561 | −0.72 | 259.0612, 243.0663, 177.0557, 151.0037, 125.0244 | 8.7 | Dihydrokaempferol ** |
| Flavanones | ||||||||
| 1fn | 11.01 | C15H12O5 | 271.0615 | 271.0612 | −1.3 | 177.0193, 151.0037, 119.0502, 107.0139 | 10.1 | Naringenin ** |
| No. | Retention Time (tr, min) | Chemical Formula | Accurate Mass [M–H]− | Exact Mass [M–H]− | Error, ppm | MS/MS Fragment Ions | Peak Area (-EIC), % of Formononetin | Compound Name |
|---|---|---|---|---|---|---|---|---|
| Isoflavones | ||||||||
| 1ir | 13.14 | C16H12O4 | 267.0670 | 267.0663 | −2.57 | 251.0350, 223.0401, 195.0452, 167.0502, 135.0088, 132.0217, 91.0189 | 100.0 | Formononetin * |
| 2ir | 11.47 | C15H10O5 | 269.0466 | 269.0455 | −3.75 | 239.0343, 223.0401, 180.0581, 159.0452, 135.0452, 133.0295, 117.0346, 107.0139, 91.0189, 63.0240 | 12.7 | Genistein * |
| 3ir | 14.05 | C16H12O5 | 283.0616 | 283.0612 | −1.42 | 267.0299, 239.0350, 223.0401, 211.0401, 195.0452, 167.0502, 132.0217 | 80.9 | Biochanin A * |
| 4ir | 10.40 | C15H10O4 | 253.0513 | 253.0506 | −2.53 | 223.0401, 208.0530, 195.0452, 180.0581, 133.0295, 132.0217, 91.0189 | 14.0 | Daidzein * |
| 5ir | 6.35 | C21H20O9 | 415.1039 | 415.1035 | −1.03 | 295.0612, 277.0506, 267.0663 | 0.4 | Puerarin * |
| Flavonoles | ||||||||
| 1fl | 12.08 | C15H10O6 | 285.0411 | 285.0405 | −2.22 | 255.0299, 239.0350, 227.0350, 211.0401, 159.0452, 143.0502, 117.0346, 93.0346 | 83.3 | Kaempferol ** |
| 2fl | 6.45 | C15H12O6 | 287.0569 | 287.0561 | −2.84 | 259.0612, 243.0663, 177.0557, 151.0037, 125.0244 | 11.1 | Dihydrokaempferol ** |
| 3fl | 11.17 | C15H10O7 | 301.0360 | 301.0354 | −2.2 | 273.0405, 257.0455, 245.0455, 229.0506, 178.9986, 151.0037, 121.0295, 107.0139 | 95.5 | Quercetin ** |
| Parameters | KF | RC |
|---|---|---|
| DPPH radical (IC50, µg/mL) | 28.17 ± 1.19 a | 42.86 ± 1.14 b |
| AAE (IC50; μg/mL) | 2.83 ± 0.004 a | 4.19 ± 0.002 b |
| TP (mg GAEe/g) | 18.4 ± 2.207 a | 13 ± 0.46 b |
| TF (mg QEe/g) | 11.5 ± 1.60 a | 6.5 ± 0.58 b |
| HOS | A-172 | Rd | A-549 | HepG2 | HEK-293 | |
|---|---|---|---|---|---|---|
| KF | 40.3 ± 1.4 a | 50.7 ± 3.0 a | 60.1 ± 4.2 a | 93.9 ± 8.1 a | 123.9 ± 11.2 a | 110.3 ± 7.2 a |
| RC | 54.9 ± 3.5 b | 82.5 ± 3.7 b | 42.4 ± 2.9 b | 92.9 ± 4.2 a | 126.4 ± 5.8 a | 107.8 ± 10.5 a |
| Cis | <4 c | <4 c | <4 c | <4 c | <4 c | <4 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboushanab, S.A.; Shevyrin, V.A.; Melekhin, V.V.; Andreeva, E.I.; Makeev, O.G.; Kovaleva, E.G. Cytotoxic Activity and Phytochemical Screening of Eco-Friendly Extracted Flavonoids from Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep and Trifolium pratense L. Flowers Using HPLC-DAD-MS/HRMS. AppliedChem 2023, 3, 119-140. https://doi.org/10.3390/appliedchem3010009
Aboushanab SA, Shevyrin VA, Melekhin VV, Andreeva EI, Makeev OG, Kovaleva EG. Cytotoxic Activity and Phytochemical Screening of Eco-Friendly Extracted Flavonoids from Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep and Trifolium pratense L. Flowers Using HPLC-DAD-MS/HRMS. AppliedChem. 2023; 3(1):119-140. https://doi.org/10.3390/appliedchem3010009
Chicago/Turabian StyleAboushanab, Saied A., Vadim A. Shevyrin, Vsevolod V. Melekhin, Elena I. Andreeva, Oleg G. Makeev, and Elena G. Kovaleva. 2023. "Cytotoxic Activity and Phytochemical Screening of Eco-Friendly Extracted Flavonoids from Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep and Trifolium pratense L. Flowers Using HPLC-DAD-MS/HRMS" AppliedChem 3, no. 1: 119-140. https://doi.org/10.3390/appliedchem3010009
APA StyleAboushanab, S. A., Shevyrin, V. A., Melekhin, V. V., Andreeva, E. I., Makeev, O. G., & Kovaleva, E. G. (2023). Cytotoxic Activity and Phytochemical Screening of Eco-Friendly Extracted Flavonoids from Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep and Trifolium pratense L. Flowers Using HPLC-DAD-MS/HRMS. AppliedChem, 3(1), 119-140. https://doi.org/10.3390/appliedchem3010009