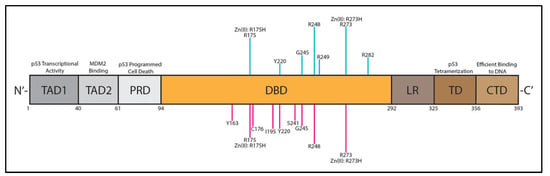
p53 Function and Dysfunction in Human Health and Diseases
(Closed)
Share This Topical Collection
Editor
 Dr. Gabriella D’Orazi
Dr. Gabriella D’Orazi
 Dr. Gabriella D’Orazi
Dr. Gabriella D’Orazi
E-Mail
Website
Collection Editor
1. Department of Neuroscience and Imaging, University G. D’Annunzio, 66013 Chieti, Italy
2. Department of Research, Unit of Cellular Network and Therapeutic Innovation, Regina Elena National Cancer Institute, 00144 Rome, Italy
Interests: tumor biology; molecular oncology; onco-suppressor p53; autophagy; hypoxia; oxidative stress; tumor microenvironment; glioblastoma; personalized medicine
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Topical Collection pertains to p53, one of the most intensively studied tumor suppressor proteins. Since its discovery, many cellular functions have been ascribed to p53, such as transcription regulation, signaling for apoptosis, involvement in the response to stress (such as genotoxic stress, the activation of oncogenes, and constitutive stress induced by factors such as hypoxia or starvation stress), senescence, DNA repair, metabolism, and immunity. The perturbation of p53 signaling pathways is required for the development of most cancers; however, it is now becoming clear that p53 can have a much broader role in human health and diseases. Thus, the activation of p53 might contribute to diseases such as neurodegenerative syndromes and also to the aging process. Much knowledge still needs to be acquired concerning the multiple contributions of this intriguing protein, not only in cancer development and the response to therapies, but also in other human diseases involving, for instance, immune dysfunction or redox state regulation. Here, we welcome papers describing recent advances in understanding p53 function and dysfunction in human diseases.
Dr. Gabriella D’Orazi
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomolecules is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- p53
- small molecules
- tumor suppressor
- apoptosis
- tumor–host interaction
- targeted therapies
- tumor microenvironment (TME)
- senescence, redox regulation
Published Papers (12 papers)
Open AccessEditorial
p53 Function and Dysfunction in Human Health and Diseases
by
Gabriella D’Orazi
Cited by 4 | Viewed by 2613
Abstract
The p53 protein is a sequence-specific DNA-binding transcription factor that, in response to stressful stimuli, regulates gene expression related to multiple cellular functions including, but not limited to, cell cycle arrest, cell apoptosis, cell growth, DNA repair, cell metabolism, and the immune response
[...] Read more.
The p53 protein is a sequence-specific DNA-binding transcription factor that, in response to stressful stimuli, regulates gene expression related to multiple cellular functions including, but not limited to, cell cycle arrest, cell apoptosis, cell growth, DNA repair, cell metabolism, and the immune response [...]
Full article
Open AccessEditor’s ChoiceReview
The Challenges and Prospects of p53-Based Therapies in Ovarian Cancer
by
Bryce Wallis, Katherine Redd Bowman, Phong Lu and Carol S. Lim
Cited by 20 | Viewed by 6697
Abstract
It has been well established that mutations in the tumor suppressor gene, p53, occur readily in a vast majority of cancer tumors, including ovarian cancer. Typically diagnosed in stages three or four, ovarian cancer is the fifth leading cause of death in women,
[...] Read more.
It has been well established that mutations in the tumor suppressor gene, p53, occur readily in a vast majority of cancer tumors, including ovarian cancer. Typically diagnosed in stages three or four, ovarian cancer is the fifth leading cause of death in women, despite accounting for only 2.5% of all female malignancies. The overall 5-year survival rate for ovarian cancer is around 47%; however, this drops to an abysmal 29% for the most common type of ovarian cancer, high-grade serous ovarian carcinoma (HGSOC). HGSOC has upwards of 96% of cases expressing mutations in p53. Therefore, wild-type (WT) p53 and p53-based therapies have been explored as treatment options via a plethora of drug delivery vehicles including nanoparticles, viruses, polymers, and liposomes. However, previous p53 therapeutics have faced many challenges, which have resulted in their limited translational success to date. This review highlights a selection of these historical p53-targeted therapeutics for ovarian cancer, why they failed, and what the future could hold for a new generation of this class of therapies.
Full article
►▼
Show Figures
Open AccessArticle
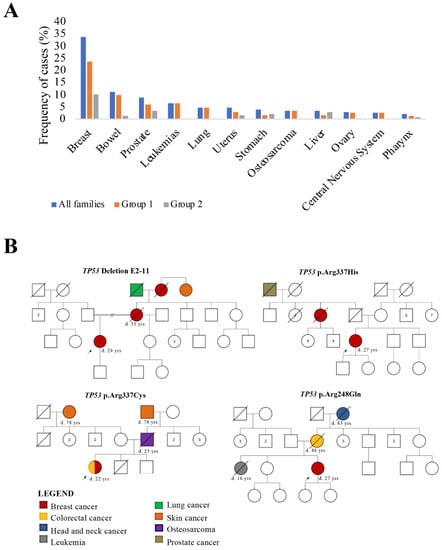
TP53 Pathogenic Variants in Early-Onset Breast Cancer Patients Fulfilling Hereditary Breast and Ovary Cancer and Li-Fraumeni-like Syndromes
by
Paula Francinete Faustino da Silva, Rebeca Mota Goveia, Thaís Bomfim Teixeira, Bruno Faulin Gamba, Aliny Pereira de Lima, Sílvia Regina Rogatto and Elisângela de Paula Silveira-Lacerda
Cited by 2 | Viewed by 3247
Abstract
TP53 gene mutation is the most common genetic alteration in human malignant tumors and is mainly responsible for Li-Fraumeni syndrome. Among the several cancers related to this syndrome, breast cancer (BC) is the most common. The
TP53 p.R337H germline pathogenic variant is highly
[...] Read more.
TP53 gene mutation is the most common genetic alteration in human malignant tumors and is mainly responsible for Li-Fraumeni syndrome. Among the several cancers related to this syndrome, breast cancer (BC) is the most common. The
TP53 p.R337H germline pathogenic variant is highly prevalent in Brazil’s South and Southeast regions, accounting for 0.3% of the general population. We investigated the prevalence of
TP53 germline pathogenic variants in a cohort of 83 BC patients from the Midwest Brazilian region. All patients met the clinical criteria for hereditary breast and ovarian cancer syndrome (HBOC) and were negative for
BRCA1 and
BRCA2 mutations. Moreover, 40 index patients fulfilled HBOC and the Li-Fraumeni-like (LFL) syndromes criteria. The samples were tested using next generation sequencing for
TP53. Three patients harbored
TP53 missense pathogenic variants (p.Arg248Gln, p.Arg337His, and p.Arg337Cys), confirmed by Sanger sequencing. One (1.2%) patient showed a large
TP53 deletion (exons 2–11), which was also confirmed. The p.R337H variant was detected in only one patient. In conclusion, four (4.8%) early-onset breast cancer patients fulfilling the HBOC and LFL syndromes presented
TP53 pathogenic variants, confirming the relevance of genetic tests in this group of patients. In contrast to other Brazilian regions,
TP53 p.R337H variant appeared with low prevalence.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Advanced Strategies for Therapeutic Targeting of Wild-Type and Mutant p53 in Cancer
by
Shengliang Zhang, Lindsey Carlsen, Liz Hernandez Borrero, Attila A. Seyhan, Xiaobing Tian and Wafik S. El-Deiry
Cited by 32 | Viewed by 7031
Abstract
TP53 is a tumor suppressor gene that encodes a sequence-specific DNA-binding transcription factor activated by stressful stimuli; it upregulates target genes involved in growth suppression, cell death, DNA repair, metabolism, among others.
TP53 is the most frequently mutated gene in tumors, with mutations
[...] Read more.
TP53 is a tumor suppressor gene that encodes a sequence-specific DNA-binding transcription factor activated by stressful stimuli; it upregulates target genes involved in growth suppression, cell death, DNA repair, metabolism, among others.
TP53 is the most frequently mutated gene in tumors, with mutations not only leading to loss-of-function (LOF), but also gain-of-function (GOF) that promotes tumor progression, and metastasis. The tumor-specific status of mutant p53 protein has suggested it is a promising target for cancer therapy. We summarize the current progress of targeting wild-type and mutant p53 for cancer therapy through biotherapeutic and biopharmaceutical methods for (1) boosting p53 activity in cancer, (2) p53-dependent and p53-independent strategies for targeting p53 pathway functional restoration in p53-mutated cancer, (3) targeting p53 in immunotherapy, and (4) combination therapies targeting p53, p53 checkpoints, or mutant p53 for cancer therapy.
Full article
►▼
Show Figures
Open AccessArticle
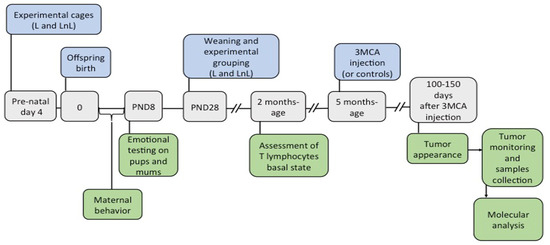
Early Social Enrichment Modulates Tumor Progression and p53 Expression in Adult Mice
by
Silvia Middei, Ludovica Giorgini, Valentina Vacca, Francesca Storri, Sabrina Putti, Georgios Strimpakos, Marcello Raspa, Ferdinando Scavizzi, Fabiola Moretti and Francesca R. D’Amato
Cited by 1 | Viewed by 2283
Abstract
Epidemiological evidence indicates that stress and aversive psychological conditions can affect cancer progression, while well-being protects against it. Although a large set of studies have addressed the impact of stress on cancer, not much is known about the mechanisms that protect from cancer
[...] Read more.
Epidemiological evidence indicates that stress and aversive psychological conditions can affect cancer progression, while well-being protects against it. Although a large set of studies have addressed the impact of stress on cancer, not much is known about the mechanisms that protect from cancer in healthy psychological conditions. C57BL/6J mouse pups were exposed to an environmental enrichment condition consisting of being raised until weaning by the biological lactating mother plus a non-lactating virgin female (LnL = Lactating and non-Lactating mothers). The Control group consisted of mice raised by a single lactating mother (L = Lactating). Four months after weaning, mice from LnL and L conditions were exposed to intramuscular injection of 3-methylcolantrene (3MCA), a potent tumorigenic drug, and onset and progression of 3MCA-induced fibrosarcomas were monitored over time. Pups from the LnL compared to the L group received more parental care and were more resilient to stressful events during the first week of life. In association, the onset of tumors in LnL adults was significantly delayed. At the molecular level, we observed increased levels of wild-type p53 protein in tumor samples of LnL compared to L adults and higher levels of its target p21 in healthy muscles of LnL mice compared to the L group, supporting the hypothesis of potential involvement of p53 in tumor development. Our study sustains the model that early life care protects against tumor susceptibility.
Full article
►▼
Show Figures
Open AccessCommunication
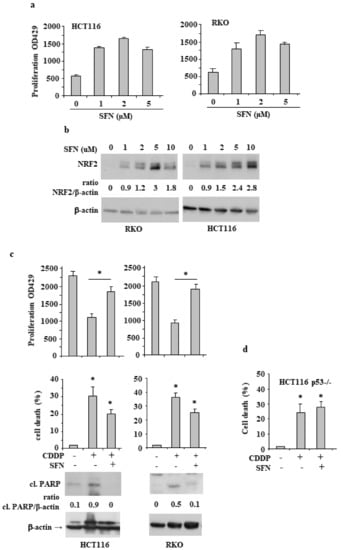
The Impact of NRF2 Inhibition on Drug-Induced Colon Cancer Cell Death and p53 Activity: A Pilot Study
by
Alessia Garufi, Giuseppa Pistritto, Valerio D’Orazi, Mara Cirone and Gabriella D’Orazi
Cited by 21 | Viewed by 3145
Abstract
Nuclear factor erythroid 2 (NF-E2) p45-related factor 2 (NRF2) protein is the master regulator of oxidative stress, which is at the basis of various chronic diseases including cancer. Hyperactivation of NRF2 in already established cancers can promote cell proliferation and resistance to therapies,
[...] Read more.
Nuclear factor erythroid 2 (NF-E2) p45-related factor 2 (NRF2) protein is the master regulator of oxidative stress, which is at the basis of various chronic diseases including cancer. Hyperactivation of NRF2 in already established cancers can promote cell proliferation and resistance to therapies, such as in colorectal cancer (CRC), one of the most lethal and prevalent malignancies in industrialized countries with limited patient overall survival due to its escape mechanisms in both chemo- and targeted therapies. In this study, we generated stable NRF2 knockout colon cancer cells (NRF2-Cas9) to investigate the cell response to chemotherapeutic drugs with regard to p53 oncosuppressor, whose inhibition we previously showed to correlate with NRF2 pathway activation. Here, we found that NRF2 activation by sulforaphane (SFN) reduced cisplatin (CDDP)-induced cell death only in NRF2-proficient cells (NRF2-ctr) compared to NRF2-Cas9 cells. Mechanistically, we found that NRF2 activation protected NRF2-ctr cells from the drug-induced DNA damage and the apoptotic function of the unfolded protein response (UPR), in correlation with reduction of p53 activity, effects that were not observed in NRF2-Cas9 cells. Finally, we found that ZnCl
2 supplementation rescued the cisplatin cytotoxic effects, as it impaired NRF2 activation, restoring p53 activity. These findings highlight NRF2′s key role in neutralizing the cytotoxic effects of chemotherapeutic drugs in correlation with reduced DNA damage and p53 activity. They also suggest that NRF2 inhibition could be a useful strategy for efficient anticancer chemotherapy and support the use of ZnCl
2 to inhibit NRF2 pathway in combination therapies.
Full article
►▼
Show Figures
Open AccessReview
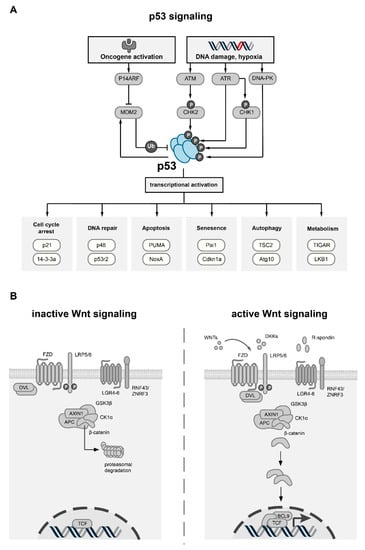
Cross-Talk between p53 and Wnt Signaling in Cancer
by
Qiyun Xiao, Johannes Werner, Nachiyappan Venkatachalam, Kim E. Boonekamp, Matthias P. Ebert and Tianzuo Zhan
Cited by 20 | Viewed by 5423
Abstract
Targeting cancer hallmarks is a cardinal strategy to improve antineoplastic treatment. However, cross-talk between signaling pathways and key oncogenic processes frequently convey resistance to targeted therapies. The p53 and Wnt pathway play vital roles for the biology of many tumors, as they are
[...] Read more.
Targeting cancer hallmarks is a cardinal strategy to improve antineoplastic treatment. However, cross-talk between signaling pathways and key oncogenic processes frequently convey resistance to targeted therapies. The p53 and Wnt pathway play vital roles for the biology of many tumors, as they are critically involved in cancer onset and progression. Over recent decades, a high level of interaction between the two pathways has been revealed. Here, we provide a comprehensive overview of molecular interactions between the p53 and Wnt pathway discovered in cancer, including complex feedback loops and reciprocal transactivation. The mutational landscape of genes associated with p53 and Wnt signaling is described, including mutual exclusive and co-occurring genetic alterations. Finally, we summarize the functional consequences of this cross-talk for cancer phenotypes, such as invasiveness, metastasis or drug resistance, and discuss potential strategies to pharmacologically target the p53-Wnt interaction.
Full article
►▼
Show Figures
Open AccessArticle
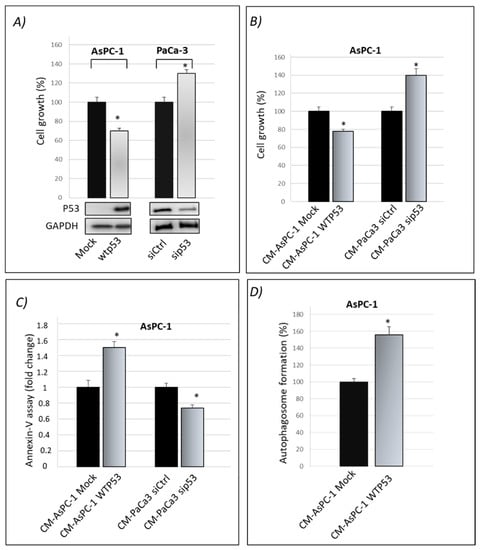
Tumor Suppressor Role of Wild-Type P53-Dependent Secretome and Its Proteomic Identification in PDAC
by
Giovanna Butera, Marcello Manfredi, Alessandra Fiore, Jessica Brandi, Raffaella Pacchiana, Veronica De Giorgis, Elettra Barberis, Virginia Vanella, Marilisa Galasso, Maria Teresa Scupoli, Emilio Marengo, Daniela Cecconi and Massimo Donadelli
Cited by 5 | Viewed by 3481
Abstract
The study of the cancer secretome is gaining even more importance in cancers such as pancreatic ductal adenocarcinoma (PDAC), whose lack of recognizable symptoms and early detection assays make this type of cancer highly lethal. The wild-type p53 protein, frequently mutated in PDAC,
[...] Read more.
The study of the cancer secretome is gaining even more importance in cancers such as pancreatic ductal adenocarcinoma (PDAC), whose lack of recognizable symptoms and early detection assays make this type of cancer highly lethal. The wild-type p53 protein, frequently mutated in PDAC, prevents tumorigenesis by regulating a plethora of signaling pathways. The importance of the p53 tumor suppressive activity is not only primarily involved within cells to limit tumor cell proliferation but also in the extracellular space. Thus, loss of p53 has a profound impact on the secretome composition of cancer cells and marks the transition to invasiveness. Here, we demonstrate the tumor suppressive role of wild-type p53 on cancer cell secretome, showing the anti-proliferative, apoptotic and chemosensitivity effects of wild-type p53 driven conditioned medium. By using high-resolution SWATH-MS technology, we characterized the secretomes of p53-deficient and p53-expressing PDAC cells. We found a great number of secreted proteins that have known roles in cancer-related processes, 30 of which showed enhanced and 17 reduced secretion in response to p53 silencing. These results are important to advance our understanding on the link between wt-p53 and cancer microenvironment. In conclusion, this approach may detect a secreted signature specifically driven by wild-type p53 in PDAC.
Full article
►▼
Show Figures
Open AccessArticle
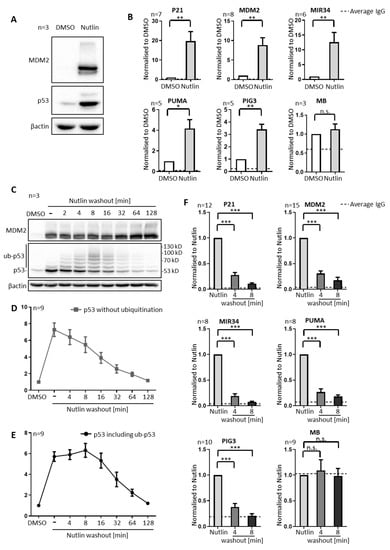
MDM2-Driven Ubiquitination Rapidly Removes p53 from Its Cognate Promoters
by
Kester Mo Henningsen, Valentina Manzini, Anna Magerhans, Sabrina Gerber and Matthias Dobbelstein
Cited by 13 | Viewed by 4407
Abstract
MDM2 is the principal antagonist of the tumor suppressor p53. p53 binds to its cognate DNA element within promoters and activates the transcription of adjacent genes. These target genes include MDM2. Upon induction by p53, the MDM2 protein binds and ubiquitinates p53, triggering
[...] Read more.
MDM2 is the principal antagonist of the tumor suppressor p53. p53 binds to its cognate DNA element within promoters and activates the transcription of adjacent genes. These target genes include MDM2. Upon induction by p53, the MDM2 protein binds and ubiquitinates p53, triggering its proteasomal degradation and providing negative feedback. This raises the question whether MDM2 can also remove p53 from its target promoters, and whether this also involves ubiquitination. In the present paper, we employ the MDM2-targeted small molecule Nutlin-3a (Nutlin) to disrupt the interaction of MDM2 and p53 in three different cancer cell lines: SJSA-1 (osteosarcoma), 93T449 (liposarcoma; both carrying amplified MDM2), and MCF7 (breast adenocarcinoma). Remarkably, removing Nutlin from the culture medium for less than five minutes not only triggered p53 ubiquitination, but also dissociated most p53 from its chromatin binding sites, as revealed by chromatin immunoprecipitation. This also resulted in reduced p53-responsive transcription, and it occurred much earlier than the degradation of p53 by the proteasome, arguing that MDM2 removes p53 from promoters prior to and thus independent of degradation. Accordingly, the short-term pharmacological inhibition of the proteasome did not alter the removal of p53 from promoters by Nutlin washout. However, when the proteasome inhibitor was applied for several hours, depleting non-conjugated ubiquitin prior to eliminating Nutlin, this compromised the removal of DNA-bound p53, as did an E1 ubiquitin ligase inhibitor. This suggests that the ubiquitination of p53 by MDM2 is necessary for its clearance from promoters. Depleting the MDM2 cofactor MDM4 in SJSA cells did not alter the velocity by that p53 was removed from promoters upon Nutlin washout. We conclude that MDM2 antagonizes p53 not only by covering its transactivation domain and by destabilization, but also by the rapid, ubiquitin-dependent termination of p53–chromatin interactions.
Full article
►▼
Show Figures
Open AccessArticle
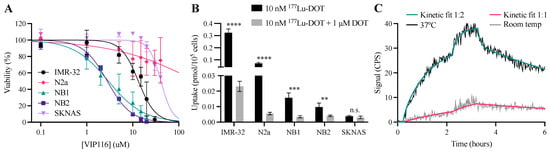
p53-Mediated Radiosensitization of 177Lu-DOTATATE in Neuroblastoma Tumor Spheroids
by
Sara Lundsten, Hanna Berglund, Preeti Jha, Cecilia Krona, Mehran Hariri, Sven Nelander, David P. Lane and Marika Nestor
Cited by 6 | Viewed by 2873
Abstract
p53 is involved in DNA damage response and is an exciting target for radiosensitization in cancer. Targeted radionuclide therapy against somatostatin receptors with
177Lu-DOTATATE is currently being explored as a treatment for neuroblastoma. The aim of this study was to investigate the
[...] Read more.
p53 is involved in DNA damage response and is an exciting target for radiosensitization in cancer. Targeted radionuclide therapy against somatostatin receptors with
177Lu-DOTATATE is currently being explored as a treatment for neuroblastoma. The aim of this study was to investigate the novel p53-stabilizing peptide VIP116 in neuroblastoma, both as monotherapy and together with
177Lu-DOTATATE. Five neuroblastoma cell lines, including two patient-derived xenograft (PDX) lines, were characterized in monolayer cultures. Four out of five were positive for
177Lu-DOTATATE uptake. IC
50 values after VIP116 treatments correlated with p53 status, ranging between 2.8–238.2 μM. IMR-32 and PDX lines LU-NB-1 and LU-NB-2 were then cultured as multicellular tumor spheroids and treated with
177Lu-DOTATATE and/or VIP116. Spheroid growth was inhibited in all spheroid models for all treatment modalities. The most pronounced effects were observed for combination treatments, mediating synergistic effects in the IMR-32 model. VIP116 and combination treatment increased p53 levels with subsequent induction of p21, Bax and cleaved caspase 3. Combination treatment resulted in a 14-fold and 1.6-fold induction of MDM2 in LU-NB-2 and IMR-32 spheroids, respectively. This, together with differential MYCN signaling, may explain the varying degree of synergy. In conclusion, VIP116 inhibited neuroblastoma cell growth, potentiated
177Lu-DOTATATE treatment and could, therefore, be a feasible treatment option for neuroblastoma.
Full article
►▼
Show Figures
Open AccessArticle
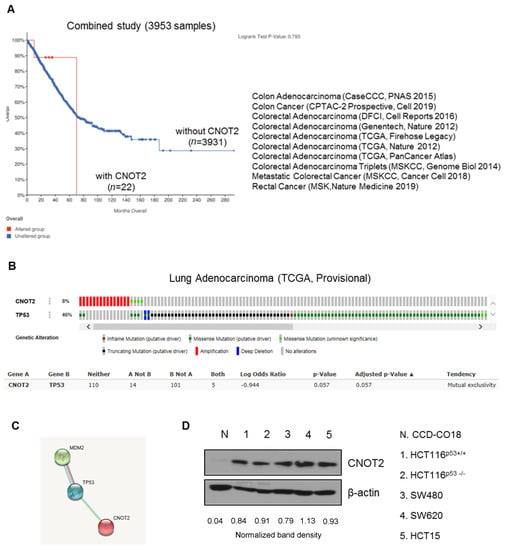
Inhibition of CNOT2 Induces Apoptosis via MID1IP1 in Colorectal Cancer Cells by Activating p53
by
Ji Hoon Jung, Duckgue Lee, Hyun Min Ko and Hyeung-Jin Jang
Cited by 13 | Viewed by 2769
Abstract
CCR4-NOT transcription complex subunit 2 (CNOT2), a subunit of the CCR4-NOT complex, has been described in cancer progression. The CNOT complex plays an important role in multiple cellular functions. Recent studies in our laboratory showed that CNOT2 promotes breast cancer cell proliferation and
[...] Read more.
CCR4-NOT transcription complex subunit 2 (CNOT2), a subunit of the CCR4-NOT complex, has been described in cancer progression. The CNOT complex plays an important role in multiple cellular functions. Recent studies in our laboratory showed that CNOT2 promotes breast cancer cell proliferation and angiogenesis. In addition, CNOT2 signals are critically related to apoptosis induced by atorvastatin in lung cancer cells. Furthermore, depletion of CNOT2 was shown to enhance the antitumor effect of midline 1 interacting protein 1 (MID1IP1) depletion, thus inhibiting c-Myc expression in liver cancer cells. However, the molecular mechanisms related to its oncogenic role remain unclear. Herein, for the first time, we report that CNOT2 inhibition can induce apoptosis in colorectal cancer cells by activating p53. Inhibition of CNOT2 markedly induced apoptosis in various cancer cells like that of the wild-type p53. Furthermore, inhibition of CNOT2 elongated p53 s half-life. Previously, our laboratory demonstrated that MID1IP1 promoted colocalization with c-Myc mediated by CNOT2. Interestingly, inhibition of CNOT2 cannot induce p53 expression without MID1IP1 or apoptosis in cancer cells. In conclusion, our results demonstrate that CNOT2 inhibition induces apoptosis through MID1IP1 by activating p53.
Full article
►▼
Show Figures
Open AccessArticle
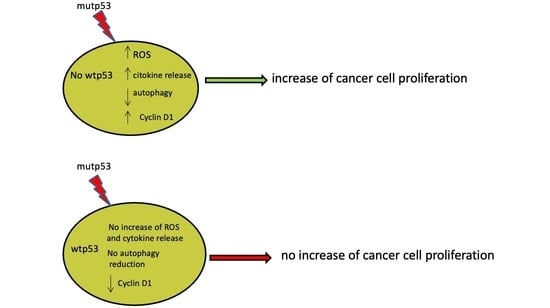
p53-R273H Sustains ROS, Pro-Inflammatory Cytokine Release and mTOR Activation While Reducing Autophagy, Mitophagy and UCP2 Expression, Effects Prevented by wtp53
by
Maria Anele Romeo, Maria Saveria Gilardini Montani, Rossella Benedetti, Andrea Arena, Gabriella D’Orazi and Mara Cirone
Cited by 6 | Viewed by 2668
Abstract
p53 is the most frequently mutated or inactivated gene in cancer, as its activity is not reconcilable with tumor onset and progression. Moreover, mutations in the
p53 gene give rise to mutant proteins such as p53-R273H that, besides losing the wild type p53
[...] Read more.
p53 is the most frequently mutated or inactivated gene in cancer, as its activity is not reconcilable with tumor onset and progression. Moreover, mutations in the
p53 gene give rise to mutant proteins such as p53-R273H that, besides losing the wild type p53 (wtp53) capacity to safeguard genome integrity, may promote carcinogenesis, mainly due to its crosstalk with pro-oncogenic pathways. Interestingly, the activation of oncogenic pathways is interconnected with reactive oxygen species (ROS) and the release of pro-inflammatory cytokines that contribute to create an inflammatory/pro-tumorigenic milieu. In this study, based on experiments involving p53-R273H silencing and transfection, we showed that this mutant p53 (mutp53) promoted cancer cell survival by increasing intracellular ROS level and pro-inflammatory/immune suppressive cytokine release, activating mTOR, reducing autophagy and mitophagy and downregulating uncoupling protein 2 (UCP2). Interestingly, p53-R273H transfection into cancer cells carrying wtp53 induced none of these effects and resulted in p21 upregulation. This suggests that wtp53 may counteract several pro-tumorigenic activities of p53-R273H and this could explain the lower aggressiveness of cancers carrying heterozygous mutp53 in comparison to those harboring homozygous mutp53.
Full article
►▼
Show Figures