Deciphering the Crosstalk between Tumor Cells and Their Microenvironment: From Molecular Aspects to Therapeutic Implications
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Tumor Microenvironment".
Viewed by 32491Editors
Interests: cancer cell biology; mechanisms of cancer cell death; hormone receptors in cancer cells; cancer stem cells; metabolic rewiring in cancer cells; drug resistance; tumor microenvironment, anticancer activity of natural compounds; prostate cancer; melanoma
Special Issues, Collections and Topics in MDPI journals
Interests: prostate cancer; melanoma; ovarian cancer; cancer biology; mechanisms of cancer cell death; cancer metastasis; cancer metabolism; cancer drug resistance; cancer stem cells; tumor microenvironment; extracellular vesicles
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
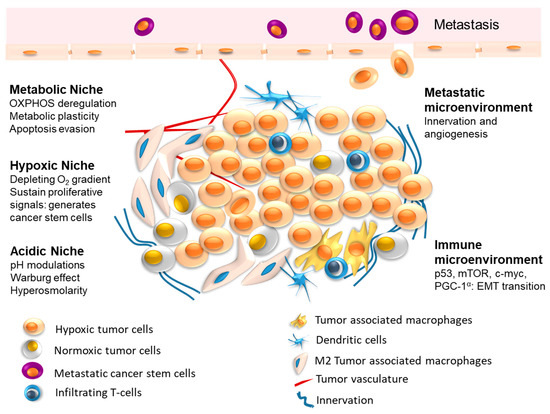
The interplay between tumor cells and their microenvironment (tumor microenvironment, TME) is a well-established hallmark feature of human tumors. TME comprises different cells, such as fibroblasts, immune cells, adipocytes, endothelial cells, as well as bone cells in distant sites of dissemination.
Cancer cells secrete biological factors to educate their neighboring cells toward an aggressive, pro-inflammatory, and pro-tumoral phenotype. In turn, cells of the TME release signals directed toward cancer cells to favor tumor growth, progression, dissemination, and treatment resistance. Interestingly, a cancer metabolic reprogramming is often involved in this pro-tumoral activity of TME cells.
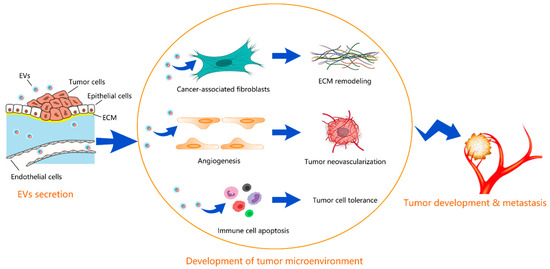
This multifaceted crosstalk is mediated by different biological factors, such as cytokines, chemokines, growth factors, extracellular matrix remodeling enzymes, as well as by extracellular vesicles (EVs). Specifically, EVs serve as transporters of different bioactive cargos, including proteins, nucleic acids, miRNAs, and lipids, from parental to both neighboring and distant target cells to participate in the various steps of malignant tumor development and progression by triggering or suppressing different signaling pathways in recipient cells.
The vicious crosstalk between cancer cells and their neighboring cells in the TME is now considered not only a prognostic/predictive biomarker of tumor growth and progression, but, more importantly, an effective target for novel and valuable therapeutic strategies.
The aim of this Collection is to invite authors to contribute original research as well as review articles highlighting the current concepts of the molecular mechanisms involved in the communication between tumor cells and their microenvironment, paving the way toward more specific and personalized medicine for cancer patients.
Prof. Dr. Patrizia Limonta
Dr. Fabrizio Fontana
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cancer cells
- tumor microenvironment (TME)
- fibroblasts
- immune cells
- adipocytes
- endothelial cells
- bone cells
- biofactors
- extracellular vesicles (EVs)
- cytokines
- chemokines
- growth factors
- hormones
- molecular cargo
- miRNAs
- precision medicine