Extracellular Vesicles and Nucleic Acids in Health and Disease
A topical collection in Cells (ISSN 2073-4409).
Viewed by 14644Editors
Interests: extracellular vesicles; lipidomics; phospholipid metabolism; cell signaling
Special Issues, Collections and Topics in MDPI journals
Interests: extracellular vesicles; autophagy; lysosomes; senescence; aging; lipidomics
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
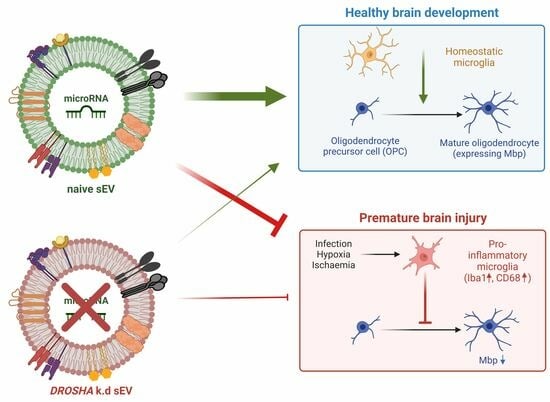
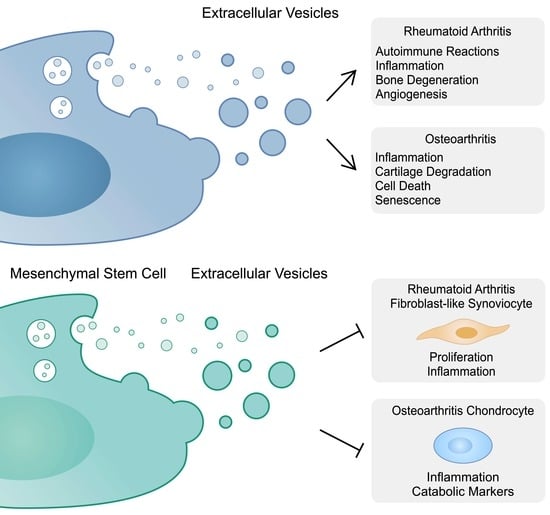
The term extracellular vesicles (EVs) is used to identify nanosized vesicles released by almost all cell types. EVs are present in every body fluid and their composition mirrors the type and the physio-pathological status of the originating cells. Due to these features and their targeting properties by recipient cells, EVs have been recently considered promising diagnostic and therapeutic tools. EVs contain different types of nucleic acids. The first studies demonstrated that EVs contain mRNAs that are translated and functionally active in target cells. This result led to the discovery that the horizontal transfer of genetic information via EVs represents one of the cell-to-cell communication mechanisms. Further, other non-coding RNA involved in protein synthesis or in regulatory functions, including tRNA, miRNA, siRNA, vtRNA, mtRNA, lncRNA, Y RNA, piRNA and snoRNA, have been found to be associated with EVs. Additionally, EVs isolated from cell culture media and biofluids carry DNA molecules, including mitochondrial DNA, single-stranded DNA and double-stranded DNA fragments.
In this Special Issue, authors are invited to submit original research and reviews on the nucleic acid content of EVs isolated from different sources and/or under different physio-pathological conditions, their biological roles, and their potential to be used as a diagnostic and therapeutic tool.
Dr. Sandra Buratta
Dr. Lorena Urbanelli
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- extracellular vesicles
- exosomes
- microvesicles
- nucleic acid delivery
- cell-free nucleic acids
- circulating RNAs
- vesicular RNAs
- nucleic acid biomarkers
- liquid biopsy